Impact of the Addition of Pyrolysed Forestry Waste to the Coking Process on the Resulting Green Biocoke
Abstract
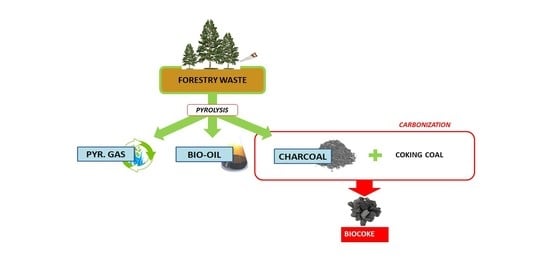
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Pyrolysis Experiments: Charcoal Production
2.3. Carbonization Experiments
2.4. Characterization of the Biomass, Charcoal and Coal Blend Samples
2.5. Characterization of the Produced Biocokes
3. Results and Discussion
3.1. Comparison of Charcoal with Commercial Reducing Agents
3.2. Production of Biocoke Formed by Mixing Charcoal with a Coking Coal Blend
3.2.1. Comparison between Charcoal and Coking Coal Blend
3.2.2. Biocokes Prepared with Different Charcoal Charges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrolysis 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D.; Gudenau, H.W. Ironmaking, 1st ed.; Verlag Stahleisen: Düsseldorf, Germany, 2016. [Google Scholar]
- Wei, R.; Zhang, L.; Cang, D.; Li, J.; Li, X.; Xu, C.C. Current status and potential of biomass utilization in ferrous metallurgical industry. Renew. Sustain. Energy Rev. 2017, 68, 511–524. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D. Coke in the iron and steel industry. In New Trends Coal Conversion: Combustion, Gasification, Emissions, and Coking; Suarez-Ruiz, I., Rubiera, F., Diez, M.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2019; pp. 367–404. [Google Scholar] [CrossRef]
- Dufourny, A.; Van de Steene, L.; Humberta, G.; Guibalb, D.; Martin, L.; Blin, J. Influence of pyrolysis conditions and the nature of the wood on the quality of charcoal as a reducing agent. J. Anal. Appl. Pyrolisis 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Kokonya, S.; Castro-Díaz, M.; Barriocanal, C.; Snape, C.E. An investigation into the effect of fast heating on fluidity development and coke quality for blends of coal and biomass. Biomass Bioenerg. 2013, 56, 295–306. [Google Scholar] [CrossRef]
- Montiano, M.G.; Díaz-Faes, E.; Barriocanal, C. Effect of briquette composition and size on the quality of the resulting coke. Fuel Process. Technol. 2016, 148, 155–162. [Google Scholar] [CrossRef]
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as blast furnace injectant—Considering availability, pretreatment and deployment in the Swedish steel industry. Energy Convers. Manag. 2015, 102, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Surup, G.R.; Leahy, J.J.; Michael, T.; Timko, M.T.; Rubetskaya, A. Hydrothermal carbonization of olive wastes to produce renewable, binder-free pellets for use as metallurgical reducing agents. Renew. Energy 2020, 155, 347–357. [Google Scholar] [CrossRef]
- Babich, A.; Arnsfeld, S.; Kowitwarangkul, P.; Senk, D. Biomass use in ironmaking: Options and limits. In Proceedings of the 6th International Congress on the Science and Technology of Ironmaking (ICSTI), Rio de Janeiro, Brazil, 14–18 October 2012; pp. 1166–1178. [Google Scholar]
- Khanna, R.; Li, K.; Wang, Z.; Sun, M.; Zhang, J.; Mukherjee, P.S. Biochars in iron and steel industries. In Char and Carbon Materials Derived from Biomass: Production, Characterization and Applications; Jeguirim, M., Limousy, L., Eds.; Elsevier Science: Mulhouse, France, 2019; pp. 429–446. [Google Scholar] [CrossRef]
- Menendez, J.A.; Alvarez, R.; Pis, J.J. Determination of metallurgical coke reactivity at INCAR: NSC and ECE-INCAR reactivity tests. Ironmak. Steelmak. 1999, 26, 117–121. [Google Scholar] [CrossRef]
- Diez, M.A.; Alvarez, R.; Barriocanal, C. Coal for metallurgical coke production: Predictions of coke quality and future requirements for cokemaking. Int. J. Coal Geol. 2002, 50, 389–412. [Google Scholar] [CrossRef]
- Díaz-Faes González, M.E. Estudio a Diferentes Escalas del Empuje de Carbones Coquizables y de la Calidad del Coque. Ph.D. Thesis, Instituto del Carbon (INCAR), University of Oviedo, Oviedo, Spain, 2010. [Google Scholar]
- Florentino-Madiedo, L.; Díaz-Faes, M.E.; Barriocanal, C. The effect of briquette composition on coking pressure generation. Fuel 2019, 258, 116–128. [Google Scholar] [CrossRef]
- Ng, K.W.; MacPhee, A.; Giroux, J.L.; Todoschuk, T. Reactivity of bio-coke with CO2. Fuel Process Technol. 2011, 92, 801–804. [Google Scholar] [CrossRef]
- MacPhee, J.A.; Gransden, J.F.; Giroux, L.; Price, J.T. Possible CO2 mitigation via addition of charcoal to coking coal blends. Fuel Process Technol. 2009, 90, 16–20. [Google Scholar] [CrossRef]
- Castro-Díaz, M.; Vega, M.F.; Díaz-Faes, E.; Barriocanal, C.; Musaa, U.; Snape, C. Evaluation of demineralized lignin and lignin-phenolic resin blends to produce biocoke suitable for blast furnace operation. Fuel 2019, 258, 116–125. [Google Scholar] [CrossRef]
- Florentino-Madiedo, L.; Díaz-Faes, E.; Barriocanal, C. Mechanical strength of bio-coke from briquettes. Renew. Energy 2020, 146, 1717–1724. [Google Scholar] [CrossRef]
- Gronli, M.; Antal, M.J. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Phounglamcheik, A.; Wang, L.; Romar, H.; Kienzl, N.; Broström, M.; Ramser, K.; Skreiberg, Ø.; Umeki, K. Effects of pyrolysis conditions and feedstocks on the properties and gasification reactivity of charcoal from woodchips. Energy Fuels 2020, 34, 8353–8365. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Septien, S.; Valin, S.; Dupont, C.; Peyrot, M.; Salvador, S. Effect of particle size and temperature on woody biomass fast pyrolysis at high temperature (1000–1400 °C). Fuel 2012, 97, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Adrados, A.; de Marco, I.; López-Urionabarrenechea, A.; Solar, J.; Caballero, B.M.; Gastelu, N. Biomass pyrolysis solids as reducing agents: Comparison with commercial reducing agents. Materials 2016, 9, 3. [Google Scholar] [CrossRef]
- Agirre, I.; Griessacher, T.; Rösler, G.; Antrekowitsch, J. Production of charcoal as an alternative reducing agent from agricultural residues using a semi-continuous semi-pilot scale pyrolysis screw reactor. Fuel Process Technol. 2013, 106, 114–121. [Google Scholar] [CrossRef]
- Griessacher, T.; Antrekowitsch, J.; Steinlechner, S. Charcoal from agricultural residues as alternative reducing agent in metal recycling. Biomass Bioenerg. 2012, 39, 139–146. [Google Scholar] [CrossRef]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of temperature and residence time in the pyrolysis of woody biomass waste in a continuous screw reactor. Biomass Bioenerg. 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Solar, J.; Hippe, F.; Babich, A.; Caballero, B.M.; de Marco, I.; Barriocanal, C.; López-Urionabarrenechea, A.; Acha, E. Conversion of injected forestry waste biomass charcoal in blast furnace: Influence of pyrolysis temperature. Energy Fuels 2021, 35, 529–538. [Google Scholar] [CrossRef]
- Solar, J.; Caballero, B.M.; de Marco, I.; López-Urionabarrenechea, A.; Gastelu, N. Optimization of charcoal production process from woody biomass waste: Effect of Ni-containing catalysts on pyrolysis vapors. Catalysts 2018, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Fassinou, W.F.; Van de Steene, L.; Toure, S.; Volle, G.; Girard, P. Pyrolysis of pinus pinaster in a two-stage gasifier: Influence of processing parameters and thermal cracking of tar. Fuel Process Technol. 2009, 90, 75–90. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Montiano, M.G.; Diaz-Faes, E.; Barriocanal, C. Partial briquetting vs direct addition of biomass in coking blends. Fuel 2014, 137, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon N. Y. 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Habibi, R.; Kopyscinski, J.; Masnadi, M.S.; Lam, J.; Grace, J.R.; Mims, C.A.; Hill, J.M. Co-gasification of biomass and non-biomass feedstocks: Synergistic and inhibition effects of switchgrass mixed with sub-bituminous coal and fluid coke during CO2 gasification. Energy Fuels 2012, 27, 494–500. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Y.; Wang, Z.; Huang, J.; Fang, Y. Interaction and its induced inhibiting or synergistic effects during co-gasification of coal char and biomass char. Bioresour. Technol. 2014, 173, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Fafard, M.; Ziegler, D.; Alamdari, H. Effects of charcoal addition on the properties of carbon anode. Metals 2017, 7, 98. [Google Scholar] [CrossRef]
- Montiano, M.G.; Barriocanal, C.; Alvarez, R. Influence of biomass on metallurgical coke quality. Fuel 2014, 116, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Flores, B.D.; Flores, I.V.; Guerrero, A.; Orellana, D.R.; Pohlmann, J.G.; Diez, M.A.; Borrego, A.G.; Osório, E.; Vilela, A.C. Effect of charcoal blending with a vitrinite rich coking coal on coke reactivity. Fuel Process Technol. 2017, 155, 97–105. [Google Scholar] [CrossRef]
- Ueki, Y.; Nunome, Y.; Yoshiie, R.; Naruse, I.; Nishibata, Y. Effect of woody biomass addition on coke properties. ISIJ Int. 2014, 54, 2454–2460. [Google Scholar] [CrossRef] [Green Version]
- Montiano, M.G.; Barriocanal, C.; Alvarez, R. Effect of the addtion of waste sawdust on the on the thermoplastic properties of a coal. Fuel 2013, 106, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, T.; Ichida, M.; Nagasaka, T.; Kato, K. Carbonization behaviour of woody biomass and resulting metallurgical coke properties. ISIJ Int. 2008, 48, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Diez, M.A.; Borrego, A.G. Evaluation of CO2-reactivity patterns in cokes from coal and woody biomass blends. Fuel 2013, 113, 59–68. [Google Scholar] [CrossRef]
- Babich, A.; Schwarz, M.; Senk, D.; Ebert, J. Influence of pre-treated biomass on the microstructure of metallurgical coke. In Proceedings of the 9th ECCC European Continuous Casting Conference, Vienna, Austria, 26–29 June 2017; pp. 1502–1507. [Google Scholar]



| Analysis | Biomass | Coal Blend |
|---|---|---|
| Moisture (wt.% as received) | 10.6 | - |
| Proximate analysis (wt.% dry basis) | ||
| Volatile matter | 79.1 | 23.2 |
| Ash | 0.7 | 9.3 |
| Fixed carbon 1 | 20.2 | 67.6 |
| Elemental analysis (wt.% dry and ash free basis) | ||
| Carbon | 47.8 | 89.7 |
| Hydrogen | 7.6 | 4.9 |
| Nitrogen | 0.0 | 1.2 |
| Sulphur | 0.0 | 0.7 |
| Others 1,2 | 44.6 | 3.5 |
| H/C ratio | 1.9 | 0.7 |
| HHV (MJ kg−1 as received) | 16.4 | - |
| Analysis | Metallurgical Coke | Petroleum Coke | Anthracite | Charcoal |
|---|---|---|---|---|
| Proximate analysis (wt.% as received/as produced basis) | ||||
| Moisture | 11.4 (<20) | 6.4 (<20) | 18.0 (<20) | 1.0 |
| Volatile matter | 3.5 (<7.0) | 9.4 (<15.0) | 5.9 (<7.0) | 5.4 |
| Ash | 11.0 (<20.0) | 1.8 (<20.0) | 9.2 (<20.0) | 3.0 |
| Fixed carbon 1 | 74.1 | 82.2 | 66.9 | 90.6 |
| HHV (MJ kg−1 as produced basis) | 26.0 | 33.6 | 25.5 | 32.3 |
| Proximate analysis (wt.% dry basis) | ||||
| Volatile matter | 3.9 (<8.75) | 10.1 (<18.75) | 7.2 (<8.75) | 5.5 |
| Ash | 12.5 (<20) | 2.0 (<20) | 11.2 (<20) | 3.0 |
| Fixed carbon 1 | 83.6 | 87.9 | 81.6 | 91.5 |
| Elemental analysis (wt.% dry basis) | ||||
| Carbon | 85.7 | 83.6 | 88.5 | 96.1 |
| Hydrogen | 0.5 | 2.8 | 0.6 | 0.2 |
| Nitrogen | 1.0 | 1.3 | 1.0 | 0.1 |
| Sulfur | 0.9 (<3.75) | 5.6 (<3.75) | 0.6 (<3.75) | 0.0 |
| Others 1,2 | 0.8 | 4.9 | 0.0 | 0.6 |
| Characteristic | Metallurgical Coke | Petroleum Coke | Anthracite | Charcoal |
|---|---|---|---|---|
| True density (g cm−3) | 1.916 | 1.389 | 1.793 | 1.798 |
| Micropore volume (cm3 g−1) | 0.01 | 0.07 | 0.05 | 0.410 |
| Surface area (m2 g−1) | 24 | 156 | 122 | 491 |
| Particle Size | Charcoal | Coal Blend |
|---|---|---|
| >5 mm | 0.0 | 5.0 |
| 4–5 mm | 0.0 | 2.1 |
| 3–4 mm | 0.0 | 4.9 |
| 2–3 mm | 0.1 | 8.5 |
| 1–2 mm | 28.6 | 15.8 |
| 0.5–1 mm | 39.0 | 23.3 |
| <0.5 mm | 32.3 | 40.4 |
| Sample | Ash | SiO2 | Al2O3 | CaO | K2O | Fe2O3 | MgO | P2O5 | SO3 | TiO2 | Na2O | MnO | Rest | AI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Charcoal | 3.0 | 32.6 | 8.4 | 25.5 | 14.2 | 3.9 | 5.1 | 4.4 | 2.3 | 0.4 | 1.2 | 1.1 | 0.8 | 0.9 |
| Coal blend | 9.3 | 54.9 | 29.1 | 2.0 | 2.9 | 5.8 | 0.8 | 0.8 | 1.2 | 1.7 | 0.5 | 0.1 | 0.3 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solar, J.; Caballero, B.M.; Barriocanal, C.; Lopez-Urionabarrenechea, A.; Acha, E. Impact of the Addition of Pyrolysed Forestry Waste to the Coking Process on the Resulting Green Biocoke. Metals 2021, 11, 613. https://doi.org/10.3390/met11040613
Solar J, Caballero BM, Barriocanal C, Lopez-Urionabarrenechea A, Acha E. Impact of the Addition of Pyrolysed Forestry Waste to the Coking Process on the Resulting Green Biocoke. Metals. 2021; 11(4):613. https://doi.org/10.3390/met11040613
Chicago/Turabian StyleSolar, Jon, Blanca María Caballero, Carmen Barriocanal, Alexander Lopez-Urionabarrenechea, and Esther Acha. 2021. "Impact of the Addition of Pyrolysed Forestry Waste to the Coking Process on the Resulting Green Biocoke" Metals 11, no. 4: 613. https://doi.org/10.3390/met11040613
APA StyleSolar, J., Caballero, B. M., Barriocanal, C., Lopez-Urionabarrenechea, A., & Acha, E. (2021). Impact of the Addition of Pyrolysed Forestry Waste to the Coking Process on the Resulting Green Biocoke. Metals, 11(4), 613. https://doi.org/10.3390/met11040613