Efficient Recovery of Rare Earth Elements (Pr(III) and Tm(III)) From Mining Residues Using a New Phosphorylated Hydrogel (Algal Biomass/PEI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
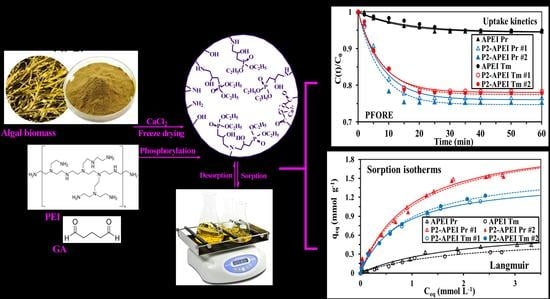
2.2. Synthesis of Sorbents
2.2.1. Synthesis of Algal/Poly(ethyleneimine) (PEI) Beads (APEI)
2.2.2. Synthesis of Activated APEI Beads (Methylene Chloride Grafted Spacer Arms)
2.2.3. Phosphorylation of APEI Activated Beads (P2-APEI)
2.3. Characterization of Materials
2.4. Sorption Tests
2.5. Treatment of Ore Residues
3. Results and Discussion
3.1. Characterization of Materials
3.1.1. Scanning Electron Microscopy (SEM) and SEM-EDX Characterizations
3.1.2. Textural Properties
3.1.3. Thermogravimetric Analysis
3.1.4. FTIR Analysis
3.1.5. XPS Analysis
3.1.6. Elemental Analysis and pHPZC
3.2. Sorption Studies on Synthetic Solutions
3.2.1. pH Effect
3.2.2. Uptake Kinetics
3.2.3. Sorption Isotherms
3.2.4. Sorption Mechanism
3.2.5. Sorption Selectivity
3.2.6. Metal Desorption and Sorbent Recycling
3.3. Application to Metal Recovery from Acid Leaching of Tailing Material
3.3.1. Pre-Treatment of Leachates
3.3.2. Metal Recovery from Pre-Treated Solutions
3.3.3. Rare Earth Elements (REEs) Separation and Precipitation as Oxalate Salts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEI | Polyethyleneimine |
| APEI | Algal-polyethyleneimine |
| APEI-Cl | Methylene chloride grafted spacer arms (APEI-with epichlorohydrin) |
| P2-APEI | Phosphorylation of algal-polyethyleneimine |
| ICP-AES | Inductively coupled plasma atomic emission spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| TGA | Thermal gravimetric analysis or thermogravimetric analysis |
| SEM | Scanning electron microscope |
| SEM-EDX | Scanning electron microscopy-energy dispersive x-ray analysis |
| BET | Brunauer-Emmett-Teller |
| DrDTG | Derivative-differential thermogravimetric analysis |
| SD | Sorbent dosage |
| PFORE | Pseudo-first order rate equation |
| PSORE | Pseudo-second order rate equation |
| RIDE | Resistance to intraparticle diffusion equation—Crank equation |
| AIC | Akaike information criterion |
| SC | selectivity coefficient |
| pHPZC | Point of zero charge |
| pH0 | Initial pH of the solution |
| pHeq | Equilibrium pH after sorption |
| Kd | Distribution ratio |
| C0 | Initial concentration |
| Ceq | Equilibrium concentration after metal sorption |
| BEs | Binding energies |
| AF | Atomic fractions |
| REEs | Rare earth elements |
| HSAB Biopolymer-LDH | Hard and Soft Acid-Base theory Biopolymer-layered double hydroxides composite |
References
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef] [Green Version]
- Wall, F. Rare earth elements. In Critical Metals Handbook; Wiley: Hoboken, NJ, USA, 2014; pp. 312–339. [Google Scholar]
- Duarte, F.J. Tunable Lasers Handbook; Elsevier Academic Press: San Diego, CA, USA, 1995; 477p. [Google Scholar]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J.; Stauffer, P.H.; Hendley, J.W. Rare Earth Elements: Critical Resources for High Technology; Fact Sheet 087-02; U.S. Geological Survey: Reston, VA, USA, 2002. [CrossRef] [Green Version]
- RSC. Periodic Table. Available online: https://www.rsc.org/periodic-table/ (accessed on 30 October 2020).
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Xu, D.; Shah, Z.; Cui, Y.; Jin, L.; Peng, X.; Zhang, H.; Sun, G. Recovery of rare earths from nitric acid leach solutions of phosphate ores using solvent extraction with a new amide extractant (TODGA). Hydrometallurgy 2018, 180, 132–138. [Google Scholar] [CrossRef]
- Yuan, H.; Hong, W.; Zhou, Y.; Pu, B.; Gong, A.; Xu, T.; Yang, Q.; Li, F.; Qiu, L.; Zhang, W.; et al. Extraction and back-extraction behaviors of 14 rare earth elements from sulfuric acid medium by TODGA. J. Rare Earths 2018, 36, 642–647. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, Z.H.; Li, D.Q.; Niu, C.J. Adsorption of heavy rare earth(III) with extraction resin containing bis(2,4,4-trimethylpentyl) monothiophosphinic acid. J. Alloys Compd. 2004, 374, 434–437. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, J.; Shen, C.; Chen, Q. Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin. Chin. J. Chem. Eng. 2012, 20, 823–830. [Google Scholar] [CrossRef]
- Yamada, E.; Murakami, H.; Nishihama, S.; Yoshizuka, K. Separation process of dysprosium and neodymium from waste neodymium magnet. Sep. Purif. Technol. 2018, 192, 62–68. [Google Scholar] [CrossRef]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.-M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Van der Hoogerstraete, T.; Binnemans, K. Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl(tetradecyl)phosphonium nitrate: A process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem. 2014, 16, 1594–1606. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Repo, E.; Meng, Y.; Wang, X.; Yin, D.; Sillanpaa, M. An EDTA-beta-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J. Coll. Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef]
- Abreu, R.D.; Morais, C.A. Study on separation of heavy rare earth elements by solvent extraction with organophosphorus acids and amine reagents. Miner. Eng. 2014, 61, 82–87. [Google Scholar] [CrossRef]
- Huang, X.; Dong, J.; Wang, L.; Feng, Z.; Xue, Q.; Meng, X. Selective recovery of rare earth elements from ion-adsorption rare earth element ores by stepwise extraction with HEH(EHP) and HDEHP. Green Chem. 2017, 19, 1345–1352. [Google Scholar] [CrossRef]
- Sharaf, M.; Yoshida, W.; Kubota, F.; Goto, M. Selective Extraction of Scandium by a Long Alkyl Chain Carboxylic Acid/Organophosphonic Ester Binary Extractant. Solvent Extr. Ion Exch. 2018, 36, 647–657. [Google Scholar] [CrossRef]
- Singh, D.K.; Anitha, M.; Kain, V. Development of solvent extraction process for erbium purification. Sep. Sci. Technol. 2017, 52, 2284–2290. [Google Scholar] [CrossRef]
- Chen, J.; Luo, W.; Guo, A.; Luo, T.; Lin, C.; Li, H.; Jing, L. Preparation of a novel carboxylate-rich palygorskite as an adsorbent for Ce3+ from aqueous solution. J. Coll. Interface Sci. 2018, 512, 657–664. [Google Scholar] [CrossRef]
- Borai, E.H.; Hamed, M.G.; El-Kamash, A.M.; Siyam, T.; Elsayed, G.O. Template polymerization synthesis of hydrogel and silica composite for sorption of some rare earth elements. J. Coll. Interface Sci. 2015, 456, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Florek, J.; Giret, S.; Juère, E.; Larivière, D.; Kleitz, F. Functionalization of mesoporous materials for lanthanide and actinide extraction. Dalton Trans. 2016, 45, 14832–14854. [Google Scholar] [CrossRef] [PubMed]
- Gargari, J.E.; Kalal, H.S.; Shakeri, A.; Khanchi, A. Synthesis and characterization of Silica/polyvinyl imidazole/H2PO4-core-shell nanoparticles as recyclable adsorbent for efficient scavenging of Sm(III) and Dy(III) from water. J. Coll. Interface Sci. 2017, 505, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Lee, Y.-R.; Yu, K.; Ahn, J.-W.; Ahn, W.-S. Benzene triamido-tetraphosphonic acid immobilized on mesoporous silica for adsorption of Nd 3+ ions in aqueous solution. Microporous Mesoporous Mater. 2018, 258, 62–71. [Google Scholar] [CrossRef]
- Maranescu, B.; Lupa, L.; Visa, A. Synthesis, characterization and rare earth elements adsorption properties of phosphonate metal organic frameworks. Appl. Surf. Sci. 2019, 481, 83–91. [Google Scholar] [CrossRef]
- Saha, D.; Akkoyunlu, S.D.; Thorpe, R.; Hensley, D.K.; Chen, J. Adsorptive recovery of neodymium and dysprosium in phosphorous functionalized nanoporous carbon. J. Environ. Chem. Eng. 2017, 5, 4684–4692. [Google Scholar] [CrossRef]
- Ang, K.L.; Li, D.; Nikoloski, A.N. The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 2. Chelating resins. Miner. Eng. 2018, 123, 8–15. [Google Scholar] [CrossRef]
- Fila, D.; Hubicki, Z.; Kołodyńska, D. Recovery of metals from waste nickel-metal hydride batteries using multifunctional Diphonix resin. Adsorption 2019, 25, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Galhoum, A.A.; Elshehy, E.A.; Tolan, D.A.; El-Nahas, A.M.; Taketsugu, T.; Nishikiori, K.; Akashi, T.; Morshedy, A.S.; Guibal, E. Synthesis of polyaminophosphonic acid-functionalized poly(glycidyl methacrylate) for the efficient sorption of La(III) and Y(III). Chem. Eng. J. 2019, 375, 121932. [Google Scholar] [CrossRef]
- Hérès, X.; Blet, V.; Di Natale, P.; Ouaattou, A.; Mazouz, H.; Dhiba, D.; Cuer, F. Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins. Metals 2018, 8, 682. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-J.; Tavlarides, L.L. Adsorption of Neodymium(III) from Aqueous Solutions Using a Phosphorus Functionalized Adsorbent. Ind. Eng. Chem. Res. 2010, 49, 12567–12575. [Google Scholar] [CrossRef]
- Radhika, S.; Nagaraju, V.; Kumar, B.N.; Kantam, M.L.; Reddy, B.R. Solid-liquid extraction of Gd(III) and separation possibilities of rare earths from phosphoric acid solutions using Tulsion CH-93 and Tulsion CH-90 resins. J. Rare Earths 2012, 30, 1270–1275. [Google Scholar] [CrossRef]
- Pinto, J.; Henriques, B.; Soares, J.; Costa, M.; Dias, M.; Fabre, E.; Lopes, C.B.; Vale, C.; Pinheiro-Torres, J.; Pereira, E. A green method based on living macroalgae for the removal of rare-earth elements from contaminated waters. J. Environ. Manag. 2020, 263, 110376. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, D.L.; Porada, S.; Sillanpää, M. Marine algae: A promising resource for the selective recovery of scandium and rare earth elements from aqueous systems. Chem. Eng. J. 2019, 371, 759–768. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Li, W.; Zhou, H.; Yang, X.; Sui, N.; Liu, H. Preparation of Several Alginate Matrix Gel Beads and their Adsorption Properties Towards Rare Earths (III). Waste Biomass Valorization 2013, 4, 665–674. [Google Scholar] [CrossRef]
- Song, D.; Park, S.-J.; Kang, H.W.; Bin Park, S.; Han, J.-I. Recovery of Lithium(I), Strontium(II), and Lanthanum(III) Using Ca–Alginate Beads. J. Chem. Eng. Data 2013, 58, 2455–2464. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Pan, F.; Zhou, H.; Yang, X.; Li, W.; Liu, H. Adsorption Properties toward Trivalent Rare Earths by Alginate Beads Doping with Silica. Ind. Eng. Chem. Res. 2013, 52, 3453–3461. [Google Scholar] [CrossRef]
- Wang, F.C.; Zhao, J.M.; Wei, X.T.; Huo, F.; Li, W.S.; Hu, Q.Y.; Liu, H.Z. Adsorption of rare earths (III) by calcium alginate-poly glutamic acid hybrid gels. J. Chem. Technol. Biotechnol. 2014, 89, 969–977. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, L.; Wang, L.; Zhu, B.; Fan, L. Adsorption of lanthanum by magnetic alginate-chitosan gel beads. J. Chem. Technol. Biotechnol. 2011, 86, 345–352. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.; Gao, Y.; Zhang, S.; Wu, K. Adsorption of rare earths(III) using an efficient sodium alginate hydrogel cross-linked with poly-gamma-glutamate. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Cataldo, S.; Gianguzza, A.; Merli, M.; Muratore, N.; Piazzese, D.; Liveri, M.L.T. Experimental and robust modeling approach for lead(II) uptake by alginate gel beads: Influence of the ionic strength and medium composition. J. Coll. Interface Sci. 2014, 434, 77–88. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, X.-F.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Coll. Interface Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Q.; Li, Z.; Liu, C.; Liu, X.; Liu, Y.; Dong, G.; Lan, T.; Wei, Y. Fabrication of efficient alginate composite beads embedded with N-doped carbon dots and their application for enhanced rare earth elements adsorption from aqueous solutions. J. Coll. Interface Sci. 2020, 562, 224–234. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Rodriguez-Castellón, E.; Guibal, E. A new method for incorporating polyethyleneimine (PEI) in algal beads: High stability as sorbent for palladium recovery and supported catalyst for nitrophenol hydrogenation. Mater. Chem. Phys. 2019, 221, 144–155. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Lu, S.; Hamza, M.F.; Fujita, T.; Vincent, T.; Guibal, E. Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions. Molecules 2019, 24, 3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, M.F.; Salih, K.A.M.; Abdel-Rahman, A.A.-H.; Zayed, Y.E.; Wei, Y.; Liang, J.; Guibal, E. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem. Eng. J. 2021, 403, 126399. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Guibal, E. Quaternization of algal/PEI beads (a new sorbent): Characterization and application to scandium recovery from aqueous solutions. Chem. Eng. J. 2020, 383, 123210. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, A. Development and validation of an adsorption kinetic model at solid-liquid interface using normalized Gudermannian function. J. Mol. Liq. 2019, 276, 67–77. [Google Scholar] [CrossRef]
- Shapiro, L. Rapid Analysis of Silicate, Carbonate, and Phosphate Rocks; Report No. 1401; U.S. Geological Survey: Reston, VA, USA, 1975; Volume 76, pp. 3–88.
- Marczenko, Z. Separation and Spectrophotometric Determination of Elements, 2nd ed.; Ellis Horwood: Chichester, UK, 1986; 678p. [Google Scholar]
- Davies, W.; Gray, W. A rapid and specific titrimetric method for the precise determination of uranium using iron(II) sulphate as reductant. Talanta 1964, 11, 1203–1211. [Google Scholar] [CrossRef]
- Nazir, R.; Gaan, S. Recent developments in P(O/S)–N containing flame retardants. J. Appl. Polym. Sci. 2020, 137, 27. [Google Scholar] [CrossRef] [Green Version]
- Demadis, K.D.; Paspalaki, M.; Theodorou, J. Controlled Release of Bis(phosphonate) Pharmaceuticals from Cationic Biodegradable Polymeric Matrices. Ind. Eng. Chem. Res. 2011, 50, 5873–5876. [Google Scholar] [CrossRef]
- Haug, A. Dissociation of alginic acid. Acta Chem. Scand. 1961, 15, 950–952. [Google Scholar] [CrossRef]
- Li, J.-N.; Liu, L.; Fu, Y.; Guo, Q.-X. What are the pKa values of organophosphorus compounds? Tetrahedron 2006, 62, 4453–4462. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Eisa, W.H.; El-Sayed, I.E.-T.; Tolba, A.A.; Shalaby, Z.M.; Mohamady, S.I.; Muhammad, S.S.; Hussien, S.S.; Akashi, T.; Guibal, E. A new route for manufacturing poly(aminophosphonic)-functionalized poly(glycidyl methacrylate)-magnetic nanocomposite—Application to uranium sorption from ore leachate. Environ. Pollut. 2020, 264, 114797. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; 414p. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Marcus, Y. Ion Properties; Marcel Dekker, Inc.: New York, NY, USA, 1997; 259p. [Google Scholar]
- Hamza, M.F.; Lu, S.M.; Salih, K.A.M.; Mira, H.; Dhmees, A.S.; Fujita, T.; Wei, Y.Z.; Vincent, T.; Guibal, E. As(V) sorption from aqueous solutions using quaternized algal/polyethyleneimine composite beads. Sci. Total. Environ. 2020, 719, 137396. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Mubark, A.E.; Wei, Y.; Vincent, T.; Guibal, E. Quaternization of Composite Algal/PEI Beads for Enhanced Uranium Sorption—Application to Ore Acidic Leachate. Gels 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Ramasamy, D.L.; Naseer, W.A.; Sillanpää, M. A novel approach for synthesis of exfoliated biopolymeric-LDH hybrid nanocomposites via in-stiu coprecipitation with gum Arabic: Application towards REEs recovery. Chem. Eng. J. 2018, 347, 398–406. [Google Scholar] [CrossRef]
- Rhauderwiek, T.; Zhao, H.; Hirschle, P.; Döblinger, M.; Bueken, B.; Reinsch, H.; De Vos, D.; Wuttke, S.; Kolb, U.; Stock, N. Highly stable and porous porphyrin-based zirconium and hafnium phosphonates—Electron crystallography as an important tool for structure elucidation. Chem. Sci. 2018, 9, 5467–5478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sum, J.Y.; Ahmad, A.L.; Ooi, B.S. Selective separation of heavy metal ions using amine-rich polyamide TFC membrane. J. Ind. Eng. Chem. 2019, 76, 277–287. [Google Scholar] [CrossRef]
- Surls, J.P.; Choppin, G.R. Equilibrium Sorption of Lanthanides, Americium and Curium on Dowex-50 Resin. J. Am. Chem. Soc. 1957, 79, 855–859. [Google Scholar] [CrossRef]
- Philip, L.; Iyengar, L.; Venkobachar, C. Biosorption of U, La, Pr, Nd, Eu and Dy by Pseudomonas aeruginosa. J. Ind. Microbiol. Biotechnol. 2000, 25, 1–7. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J. Entrapment of brown seaweeds (Turbinaria conoides and Sargassum wightii) in polysulfone matrices for the removal of praseodymium ions from aqueous solutions. J. Rare Earths 2015, 33, 1196–1203. [Google Scholar] [CrossRef]
- Varshini, J.S.; Das, D.; Das, N. Optimization of parameters for praseodymium(III) biosorption onto biowaste materials using response surface methodology: Equilibrium, kinetic and regeneration studies. Ecol. Eng. 2015, 81, 321–327. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hamza, M.F.; Vincent, T.; Faur, C.; Guibal, E. Praseodymium sorption on Laminaria digitata algal beads and foams. J. Coll. Interface Sci. 2017, 504, 780–789. [Google Scholar] [CrossRef]
- Saeed, M.M.; Ahmed, M. Effect of Temperature on Kinetics and Adsorption Profile of Endothermic Chemisorption Process: –Tm(III)–PAN Loaded PUF System. Sep. Sci. Technol. 2006, 41, 705–722. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Vijayaraghavan, K. Biosorption of Tm(III) by free and polysulfone-immobilized Turbinaria conoides biomass. J. Ind. Eng. Chem. 2019, 80, 318–324. [Google Scholar]
- Iftekhar, S.; Srivastava, V.; Ben Hammouda, S.; Sillanpää, M. Fabrication of novel metal ion imprinted xanthan gum-layered double hydroxide nanocomposite for adsorption of rare earth elements. Carbohydr. Polym. 2018, 194, 274–284. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Jorjani, E.; Shahbazi, M. The production of rare earth elements group via tributyl phosphate extraction and precipitation stripping using oxalic acid. Arab. J. Chem. 2016, 9, S1532–S1539. [Google Scholar] [CrossRef]
- Da Silva, R.G.; Morais, C.A.; Teixeira, L.V.; Oliveira, É.D. Selective Precipitation of High-Quality Rare Earth Oxalates or Carbonates from a Purified Sulfuric Liquor Containing Soluble Impurities. Min. Met. Explor. 2019, 36, 967–977. [Google Scholar] [CrossRef]
- Tunsu, C.; Lapp, J.B.; Ekberg, C.; Retegan, T. Selective separation of yttrium and europium using Cyanex 572 for applications in fluorescent lamp waste processing. Hydrometallurgy 2016, 166, 98–106. [Google Scholar] [CrossRef]













| Model | Parameter | Sorbent | ||
|---|---|---|---|---|
| APEI | P2-APEI | |||
| 1st Run | 2nd Run | |||
| Exp. | qeq,exp (mmol g−1) | 0.0795 | 0.357 | 0.348 |
| PFORE | q1,calc (mmol g−1) | 0.0828 | 0.365 | 0.356 |
| k1 × 102 (min−1) | 7.45 | 12.8 | 14.0 | |
| R2 | 0.980 | 0.985 | 0.973 | |
| AIC | −133 | −102 | −94 | |
| PSORE | q2,calc (mmol g−1) | 0.101 | 0.418 | 0.405 |
| k2 × 10 (L mmol−1 min−1) | 7.95 | 3.81 | 4.35 | |
| R2 | 0.965 | 0.956 | 0.938 | |
| AIC | −126 | −90 | −85 | |
| RIDE | De × 108 (m2 min−1) | 3.66 | 4.91 | 5.30 |
| R2 | 0.960 | 0.960 | 0.945 | |
| AIC | −124 | −92 | −87 | |
| Model | Parameter | Sorbent | ||
|---|---|---|---|---|
| APEI | P2-APEI | |||
| 1st Run | 2nd Run | |||
| Exp. | qeq,exp (mmol g−1) | 0.0608 | 0.271 | 0.269 |
| PFORE | q1,calc (mmol g−1) | 0.0666 | 0.276 | 0.275 |
| k1 × 102 (min−1) | 5.09 | 13.5 | 12.7 | |
| R2 | 0.964 | 0.992 | 0.985 | |
| AIC | −130 | −113 | −104 | |
| PSORE | q2,calc (mmol g−1) | 0.085 | 0.313 | 0.315 |
| k2 × 10 (L mmol−1 min−1) | 5.67 | 5.64 | 5.06 | |
| R2 | 0.953 | 0.969 | 0.960 | |
| AIC | −125 | −98 | −93 | |
| RIDE | De × 108 (m2 min−1) | 2.74 | 5.16 | 4.92 |
| R2 | 0.933 | 0.975 | 0.964 | |
| AIC | −122 | −101 | −95 | |
| Model | Parameter | Sorbent | |||
|---|---|---|---|---|---|
| APEI | P2-APEI | ||||
| Pr(III) | Tm(III) | Pr(III) | Tm(III) | ||
| Exp. | qeq,exp (mmol g−1) | 0.047 | 0.046 | 0.190 | 0.184 |
| PFORE | q1,calc (mmol g−1) | 0.047 | 0.047 | 0.196 | 0.186 |
| k1 × 102 (min−1) | 1.59 | 1.02 | 1.05 | 1.10 | |
| R2 | 0.987 | 0.988 | 0.988 | 0.988 | |
| AIC | −154 | −154 | −119 | −123 | |
| PSORE | q2,calc (mmol g−1) | 0.053 | 0.055 | 0.228 | 0.212 |
| k2 × 10 (L mmol−1 min−1) | 39.7 | 22.5 | 5.62 | 6.91 | |
| R2 | 0.974 | 0.978 | 0.968 | 0.982 | |
| AIC | −145 | −145 | −107 | −118 | |
| RIDE | De × 108 (m2 min−1) | 6.72 | 4.55 | 4.43 | 4.54 |
| R2 | 0.976 | 0.978 | 0.970 | 0.985 | |
| AIC | −149 | −147 | −108 | −122 | |
| Model | Parameter | Sorbent | |||||
|---|---|---|---|---|---|---|---|
| APEI | P2-APEI | ||||||
| Pr(III) | Tm(III) | Pr(III) #1 | Pr(III) #2 | Tm(III) #1 | Tm(III) #2 | ||
| Exp. | qm,exp | 0.448 | 0.331 | 1.554 | 1.525 | 1.135 | 1.230 |
| Langmuir | qm,L | 0.752 | 0.584 | 2.14 | 2.151 | 1.494 | 1.645 |
| bL | 0.502 | 0.539 | 1.05 | 0.980 | 1.334 | 1.153 | |
| R2 | 0.988 | 0.983 | 0.990 | 0.989 | 0.996 | 0.994 | |
| AIC | −81 | −84 | −55 | −54 | −70 | −67 | |
| Freundlich | kF | 0.235 | 0.191 | 1.02 | 0.998 | 0.794 | 0.820 |
| nF | 1.635 | 1.580 | 2.105 | 2.060 | 2.104 | 1.992 | |
| R2 | 0.968 | 0.966 | 0.984 | 0.985 | 0.994 | 0.995 | |
| AIC | −71 | −76 | −52 | −53 | −70 | −71 | |
| Sips | qm,S | 0.540 | 0.392 | 2.867 | 3.161 | 2.269 | 2.749 |
| bS | 0.922 | 1.21 | 0.597 | 0.488 | 0.572 | 0.447 | |
| nS | 0.666 | 0.621 | 1.319 | 1.380 | 1.422 | 1.441 | |
| R2 | 0.994 | 0.991 | 0.990 | 0.989 | 0.998 | 0.998 | |
| AIC | −84 | −83 | −53 | −53 | −80 | −80 | |
| Metal | Sorbent | pH | teq (min) | qm,L (mmol g−1) | bL (L mmol−1) | Ref. |
|---|---|---|---|---|---|---|
| Pr(III) | Pseudomonas aeruginosa | 5 | 240 | 0.94 | n.r. | [69] |
| D72 resin (–SO3H) | 3 | 1440 | 2.08 | 8.60 | [10] | |
| Turbinaria conoides | 5 | 90 | 1.04 | 7.33 | [70] | |
| T. conoides/polysulfone beads | 5 | 240 | 0.85 | 2.40 | [70] | |
| Crab shell | 5 | 35 | 0.47 | 3.66 | [71] | |
| Orange peel | 5 | 50 | 0.42 | 2.25 | [71] | |
| Laminaria digitata beads | 4 | 180 (FD) 1440 (AD) | 0.89 | 125.4 | [72] | |
| Laminaria digitata foams | 4 | 1440 | 0.79 | 111.3 | [72] | |
| APEI | 5 | 30 | 0.75 | 0.50 | This work | |
| P2-APEI | 5 | 20 | 2.14 | 1.02 | This work | |
| Tm(III) | PAN-polyurethane foam | 7.5 | 40 | 0.0083 | 51.1 | [73] |
| Turbinaria conoides | 5 | 200 | 1.19 | 17.1 | [74] | |
| T. conoides/polysulfone beads | 5 | 200 | 0.93 | 5.91 | [74] | |
| Zr-ion imprinted xanthan gum-layered double hydroxide | 4 | 80 | 0.19 | 255.1 | [75] | |
| APEI | 5 | 30 | 0.58 | 0.54 | This work | |
| P2-APEI | 5 | 20 | 1.57 | 1.24 | This work |
| Model | Parameter | Sorbent | ||
|---|---|---|---|---|
| APEI | P2-APEI | |||
| 1st Run | 2nd Run | |||
| Exp. | qeq,exp (mmol g−1) | 0.0788 | 0.356 | 0.347 |
| PFORE | q1,calc (mmol g−1) | 0.0813 | 0.365 | 0.343 |
| k1 × 10 (min−1) | 1.02 | 1.10 | 1.32 | |
| R2 | 0.980 | 0.983 | 0.962 | |
| AIC | −82 | −59 | −53 | |
| PSORE | q2,calc (mmol g−1) | 0.105 | 0.466 | 0.419 |
| k2 × 10 (L mmol−1 min−1) | 9.45 | 2.34 | 3.51 | |
| R2 | 0.987 | 0.990 | 0.976 | |
| AIC | −86 | −64 | −58 | |
| Model | Parameter | Sorbent | ||
|---|---|---|---|---|
| APEI | P2-APEI | |||
| 1st Run | 2nd Run | |||
| Exp. | qeq,exp (mmol g−1) | 0.0609 | 0.271 | 0.270 |
| PFORE | q1,calc (mmol g−1) | 0.0693 | 0.343 | 0.336 |
| k1 × 10 (min−1) | 0.756 | 0.531 | 0.553 | |
| R2 | 0.983 | 0.987 | 0.985 | |
| AIC | −87 | −65 | −64 | |
| PSORE | q2,calc (mmol g−1) | 0.0964 | 0.506 | 0.489 |
| k2 × 101 (L mmol−1 min−1) | 6.45 | 0.777 | 0.855 | |
| R2 | 0.984 | 0.987 | 0.986 | |
| AIC | −88 | −66 | −65 | |
| Sorption | Desorption | ||||
|---|---|---|---|---|---|
| Metal Ion | Cycle | SE (%) | St. Dev. (%) | DE (%) | St. Dev. (%) |
| Pr(III) | #1 | 99.1 | 0.3 | 100.0 | 0.3 |
| #2 | 97.7 | 0.6 | 100.5 | 0.5 | |
| #3 | 96.5 | 0.6 | 100.1 | 0.1 | |
| #4 | 94.2 | 0.1 | 100.1 | 0.0 | |
| #5 | 91.0 | 1.5 | 100.0 | 0.2 | |
| Tm(III) | #1 | 91.9 | 0.6 | 99.9 | 0.1 |
| #2 | 89.6 | 0.6 | 100.5 | 0.5 | |
| #3 | 87.2 | 0.5 | 100.2 | 0.6 | |
| #4 | 84.5 | 0.9 | 100.4 | 0.1 | |
| #5 | 82.7 | 0.6 | 99.7 | 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Salih, K.A.M.; Wei, Y.; Mira, H.; Abdel-Rahman, A.A.-H.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Efficient Recovery of Rare Earth Elements (Pr(III) and Tm(III)) From Mining Residues Using a New Phosphorylated Hydrogel (Algal Biomass/PEI). Metals 2021, 11, 294. https://doi.org/10.3390/met11020294
He C, Salih KAM, Wei Y, Mira H, Abdel-Rahman AA-H, Elwakeel KZ, Hamza MF, Guibal E. Efficient Recovery of Rare Earth Elements (Pr(III) and Tm(III)) From Mining Residues Using a New Phosphorylated Hydrogel (Algal Biomass/PEI). Metals. 2021; 11(2):294. https://doi.org/10.3390/met11020294
Chicago/Turabian StyleHe, Chunlin, Khalid A.M. Salih, Yuezhou Wei, Hamed Mira, Adel A.-H. Abdel-Rahman, Khalid Z. Elwakeel, Mohammed F. Hamza, and Eric Guibal. 2021. "Efficient Recovery of Rare Earth Elements (Pr(III) and Tm(III)) From Mining Residues Using a New Phosphorylated Hydrogel (Algal Biomass/PEI)" Metals 11, no. 2: 294. https://doi.org/10.3390/met11020294
APA StyleHe, C., Salih, K. A. M., Wei, Y., Mira, H., Abdel-Rahman, A. A. -H., Elwakeel, K. Z., Hamza, M. F., & Guibal, E. (2021). Efficient Recovery of Rare Earth Elements (Pr(III) and Tm(III)) From Mining Residues Using a New Phosphorylated Hydrogel (Algal Biomass/PEI). Metals, 11(2), 294. https://doi.org/10.3390/met11020294