Alternative Liquid-Assisted Sintering of Al/Cu Composites Using Selected Powders of As-Cast Al-Zn Alloy
Abstract
:1. Introduction
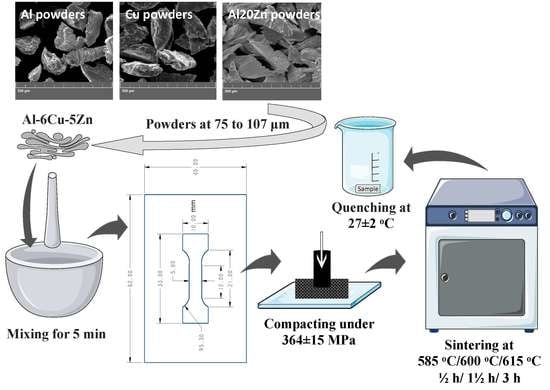
2. Materials and Methods
3. Results and Discussion
3.1. Powder Characterization
3.2. Microstructural and Mechanical Properties
3.3. Natural Aging Results
4. Conclusions
- The selection of the particle sizes provided powder sizes ranging between 200 and 140 mesh; with these sizes, the best UTS values were attained.
- Regarding this same particle size, when associated with a compaction pressure of 364 MPa, a densification level of approximately 85% was attained.
- From the separate sintering temperatures and times, it was found that UTS of 125 MPa was reached when sintering at 615 °C for 3 h is used. This value is similar to that of the as-cast Al-Cu-based alloys (produced without heat treatment). Additionally, it is also suggested that the hardness, after natural aging for 500 days, reached approximately 99 HV. With these attained mechanical properties, the Al and Cu elemental powders are associated with Al-Zn powder to constitute an Al6Cu5Zn alloy with some advantages, such as low energy consumption and a reduction in the metal fumes commonly produced by foundries.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stojanovic, B.; Bukvic, M.; Epler, I. Application of aluminum and aluminum alloys in engineering. Appl. Eng. Lett. 2018, 3, 52–56. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, K.; Frankel, G.S. Intermetallic phases in aluminum alloys and their roles in localized corrosion. J. Electrochem. Soc. 2018, 165, C807–C820. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, B. Effect of compaction pressure and sintering temperature on the liquid phase sintering behavior of Al−Cu−Zn alloy. Arch. Metall. Mater. 2015, 60, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Chak, V.; Chattopadhyay, H.; Dora, T.L. A review on fabrication methods, reinforcements and mechanical properties of aluminum matrix composites. J. Manuf. Process. 2020, 56, 1059–1074. [Google Scholar] [CrossRef]
- Bonatti, R.S.; Siqueira, R.R.; Padilha, G.S.; Bortolozo, A.D.; Osório, W.R. Distinct Alp/Sip composites affecting its densification and mechanical behavior. J. Alloys Compd. 2018, 757, 434–447. [Google Scholar] [CrossRef]
- Özay, Ç.; Gencer, E.B.; Ökçe, G. Microstructural properties of sintered Al−Cu−Mg−Sn alloys. J. Therm. Anal. Calorim. 2018, 134, 23–33. [Google Scholar] [CrossRef]
- Naeem, H.T.; Mohammed, K.S.; Ahmad, K.R. The role of cobalt and nickel intermetallic phases on the mechanical properties and microstructure evolution of Al−Zn−Mg−Cu alloys. Mater. Res. 2014, 17, 1663–1676. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.-M.; Schmid-Fetzer, R. Thermodynamic assessment of the Al–Cu–Zn system, part II: Al–Cu binary system. Calphad Comput. Coupling Phase Diagr. Thermochem. 2015, 51, 252–260. [Google Scholar] [CrossRef]
- Hou, W.T.; Shi, J.T.; Hou, L.G.; Zhang, J.S. An improved thermo−mechanical treatment of high-strength Al–Zn–Mg–Cu alloy for effective grain refinement and ductility modification. J. Mater. Process. Technol. 2017, 239, 303–314. [Google Scholar]
- Rudianto, H.; Jang, G.J.; Yang, S.S.; Kim, Y.J.; Dlouhy, I. Evaluation of sintering behavior of premix Al−Zn−Mg−Cu alloy powder. Adv. Mater. Sci. Eng. 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy; ASM International: Metals Park, OH, USA, 1984. [Google Scholar]
- Martin, J.W. Precipitation Hardening, 2nd ed.; Butterworth−Heinemann: Oxford, UK, 1998. [Google Scholar]
- Byrne, J.G.; Fine, M.E.; Kelly, A. Precipitate hardening in an aluminium−copper alloy. Philos. Mag. J. Theor. Exp. Appl. Phys. 1961, 6, 1119–1145. [Google Scholar] [CrossRef]
- Poole, W.J.; Embury, J.D.; Lloyd, D.J. Fundamentals of Aluminium Metallurgy; Woodhead Publishing Series in Metal and Surface Engineering; Woodhead Publishing: Thorston, UK, 2011; pp. 307–344. [Google Scholar]
- Ngugi, J.; Rading, G.O.; Odera, B.O.; Ngobe, B.; Forbes, R.; Cornish, L.A. Partial isothermal sections of the Cu−rich corner of the Al−Cu−Zn system at 200 and 240 °C. J. Phase Equilibria Diffus. 2019, 40, 588–599. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the influences of alloying elements on the microstructure and mechanical properties of aluminum alloys and aluminum alloy composites. Int. J. Sci. Res. 2012, 2, 1–7. [Google Scholar]
- Pournaderi, S.; Akhlaghi, F. Wear behaviour of Al6061−Al2O3 composites produced by in-situ powder metallurgy (IPM). Powder Technol. 2017, 313, 184–190. [Google Scholar] [CrossRef]
- Elias, A.L.P.; Koizumi, M.S.; Ortiz, E.L.; Rodrigues, J.F.Q.; Bortolozo, A.D.; Osório, W.R.; Padilha, G.S. Corrosion behavior of an Al−Si casting and a sintered Al/Si composite immersed into biodiesel and blends. Fuel Process. Technol. 2020, 202, 106360. [Google Scholar] [CrossRef]
- Mostafaei, A.; De Vecchis, P.R.; Nettleship, V.; Chmielus, M. Effect of powder size distribution on densification and microstructural evolution of binder−jet 3D−printed alloy 625. Mater. Des. 2019, 162, 375–383. [Google Scholar] [CrossRef]
- Hafizpour, H.R.; Simchi, A. Investigation on compressibility of Al–SiC composite powders. Powder Metall. 2008, 51, 217–223. [Google Scholar] [CrossRef]
- Bouvard, D. Densification behaviour of mixtures of hard and soft powders under pressure. Powder Technol. 2000, 111, 231–239. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Bonatti, R.S.; Costa, D.; Bortolozo, A.D.; Osório, W.R. Compaction pressure and Si content effects on compressive strengths of Al/Si/Cu alloy composites. Mater. Sci. Eng. A 2020, 770, 1385747. [Google Scholar] [CrossRef]
- Satizabal, L.M.; Caurin, H.F.N.; Meyer, Y.A.; Padilha, G.P.; Bortolozo, A.D.; Osório, W.R. Distinct heat treatments and powder size ratios affecting mechanical behavior of Al/Si/Cu composites. J. Compos. Mater. 2021, 55, 3589–3605. [Google Scholar] [CrossRef]
- Su, K.P.; Chen, X.X.; Wang, O.; Huo, D.X.; Liu, Z.W. Effect of milling on the structure and magnetic properties in Mn54Al46 flakes prepared by surfactant−assisted ball milling. Mater. Charact. 2016, 114, 263–266. [Google Scholar] [CrossRef]
- Pulido-Suárez, P.A.; Uñate-Gonzälez, K.S.; Tirado-González, J.G.; Esquerra-Arce, A.; Esquerra-Arce, J. The evolution of the microstructure and properties of ageable Al−Si−Zn−Mg alloy during the recycling of milling chips through powder metallurgy. J. Mater. Res. Technol. 2020, 9, 11769–11777. [Google Scholar] [CrossRef]
- Lumley, R.N.; Schaffer, G.B. The effect of additive particle size on the mechanical properties of sintered aluminium−copper alloys. Scr. Mater. 1998, 39, 1089–1094. [Google Scholar] [CrossRef]
- Gogola, P.; Gabalcová, Z.; Suchánek, H.; Babinec, M.; Bonek, M.; Kusý, M. Quantitative X−ray diffraction analysis of Zn−Al based alloys. Arch. Metall. Mater. 2020, 65, 959–966. [Google Scholar]
- Ogel, B.; Gurbuz, R. Microstructural characterization and tensile properties of hot pressed Al–SiC composites prepared from pure Al and Cu powders. Mater. Sci. Eng. A 2001, 301, 213–220. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Balogun, S.A.; Osoba, L.O.; Ayoola, W.A.; Oladoye, A.M. Effect of Cu and Zn addition on the mechanical properties of structural aluminum alloy. Int. J. Mod. Manuf. Technol. 2011, 3, 103–110. [Google Scholar]
- Engin, S.; Büyük, U.; Maraşli, N. The effects of microstructure and growth rate on microhardness, tensile strength, and electrical resistivity for directionally solidified Al-Ni-Fe alloys. J. Alloys Compd. 2016, 660, 23–31. [Google Scholar] [CrossRef]
- Matli, P.R.; Fareeha, U.; Shakoor, R.A.; Mohamed, A.M.A. A comparative study of structural and mechanical properties of Al−Cu composites prepared by vacuum and microwave sintering techniques. J. Mater. Res. Technol. 2018, 7, 165–172. [Google Scholar] [CrossRef]
- Ebhota, W.S.; Jen, T.-C. Formation and Their Effect on Mechanical Properties of Al−Si−X Alloys. In Intermetallic Compounds–Formation and Applications; Aliofkhazraei, M., Ed.; BoD–Books on Demand: Nordstedt, Germany, 2018. [Google Scholar]
- Zhao, J.; Liu, Z.; Bai, S.; Zeng, D.; Luo, L.; Wang, L. Effects of natural aging on the formation and strengthening effect of G.P. zones in a retrogression and re−aged Al−Zn−Mg−Cu alloy. J. Alloys Compd. 2020, 829, 154469. [Google Scholar] [CrossRef]
- Ogura, T.; Hirosawa, S.; Cerezo, A.; Sato, T. Atom probe tomography of nanoscale microstructures within precipitate free zones in Al−Zn−Mg(−Ag) alloys. Acta Mater. 2010, 58, 5714–5723. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G. Secondary ageing in an aluminum alloy 7050. Mater. Sci. Eng. A 2008, 25, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; Xin, X.; Dong, D.Y.; Ren, Y.P.; Hao, S.M. Study on the stability of the T’ phase in the Al−Zn−Cu ternary system. Acta Metall. Sin. 2004, 17, 269–273. [Google Scholar]
- Abolfazl, A.; Taheri, A.K.; Taheri, K.K. Recent advances in ageing of 7xxx series aluminum alloys: A physical metallurgy perspective. J. Alloys Compd. 2019, 781, 945–983. [Google Scholar]
- Zobac, O.; Kroupa, A.; Richter, K.W. Experimental study of the Al–Cu–Zn ternary phase diagram. J. Mater. Sci. 2020, 55, 10796–10810. [Google Scholar] [CrossRef]
- Villegas−Cardenas, J.D.; Saucedo−Muñoz, M.L.; Lopez−Hirata, V.M.; Dorates−Rosales, H.J.; Gonzalez-Velazquez, J.L. Effect of phase transformations on hardness in Zn−Al−Cu alloys. Mater. Res. 2014, 17, 1137–1144. [Google Scholar] [CrossRef]
- Casting. In ASM Handbook; ASM International: Almere, The Netherlands, 2008; Volume 15, p. 105.
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification; Trans Tech Public: Aedermannsdorf, Switzerland, 1992. [Google Scholar]
- Shabestari, S.G.; Moemeni, H. Effect of copper and solidification conditions on the microstructure and mechanical properties of Al–Si–Mg alloys. J. Mater. Processing Technol. 2004, 153−154, 193–198. [Google Scholar] [CrossRef]
- Xu, D.F.; Chen, K.H.; Chen, Y.Q.; Chen, S.Y. Evolution of the second−phase particles and their effect on tensile fracture behavior of 2219 Al−xCu alloys. Metals 2020, 10, 197. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.X.; Li, Y.D.; Liu, W.J.; Yang, H.K.; Cao, Y.J.; Bi, G.L. Effect of La and Sc Co−addition on the mechancial properties and termal conductivity of as−cast Al−4.8% Cu alloys. Metals 2021, 11, 1866. [Google Scholar] [CrossRef]
- Kang, H.J.; Park, J.Y.; Choi, Y.S.; Cho, D.H. Influence of the solution and artificial aging treatments on the microsturcture and mechanical properties of die−cast Al−Si−Mg alloys. Metals 2022, 12, 71. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, E.L.; Osório, W.R.; Bortolozo, A.D.; Padilha, G.S. Alternative Liquid-Assisted Sintering of Al/Cu Composites Using Selected Powders of As-Cast Al-Zn Alloy. Metals 2022, 12, 962. https://doi.org/10.3390/met12060962
Ortiz EL, Osório WR, Bortolozo AD, Padilha GS. Alternative Liquid-Assisted Sintering of Al/Cu Composites Using Selected Powders of As-Cast Al-Zn Alloy. Metals. 2022; 12(6):962. https://doi.org/10.3390/met12060962
Chicago/Turabian StyleOrtiz, Eder L., Wislei R. Osório, Ausdinir D. Bortolozo, and Giovana S. Padilha. 2022. "Alternative Liquid-Assisted Sintering of Al/Cu Composites Using Selected Powders of As-Cast Al-Zn Alloy" Metals 12, no. 6: 962. https://doi.org/10.3390/met12060962
APA StyleOrtiz, E. L., Osório, W. R., Bortolozo, A. D., & Padilha, G. S. (2022). Alternative Liquid-Assisted Sintering of Al/Cu Composites Using Selected Powders of As-Cast Al-Zn Alloy. Metals, 12(6), 962. https://doi.org/10.3390/met12060962