1. Introduction
Magnesium and its alloys are receiving interest as possible materials for degradable implants. Magnesium is a biodegradable and biocompatible metal; it is involved in numerous biochemical reactions in the human body, such as bone regeneration and cardiovascular protection [
1]. Moreover, one anti-inflammatory effect of Mg
2+ ions was reported as the ability to promote the M2 polarization of macrophages [
1,
2]. Furthermore, magnesium and its alloys have an elastic modulus (41–45 GPa) closer to one of the human bones than stainless steel and titanium, being suitable for avoiding stress shielding phenomena [
1,
3]. Moreover, Mg
2+ ions are involved in osteogenic differentiation, angiogenesis, limitation of osteoclastogenesis, and improvement of hydroxyapatite properties (substituting Ca
2+ ions) [
1], making them promising candidates for bone contact applications, especially temporary ones. Biodegradable metals are also of interest for cardiovascular stents, where prosthesis support is required for the time needed for arterial remodeling (typically 6–12 months) [
4]. No significant benefit can be obtained after this period, but some complications can occur (e.g., long-term endothelial dysfunction, delayed re-endothelialization, thrombogenicity, permanent physical irritation, chronic inflammatory local reactions), making the development of suitable degradable stents particularly interesting [
4].
For orthopedic and cardiovascular applications, the main limitations to the diffusion of magnesium-based degradable implants are too fast degradation combined with hydrogen gas development and significant pH increase, which cause the premature loss of mechanical requirements. In addition, some inflammatory and potentially dangerous biological effects are connected to fast degradation [
1,
3,
4]. Mg
2+ ions enrich the intracellular content by being renally controlled, as shown in the case of the oral administration of magnesium. On the other side, biodegradation of magnesium implants produces various decomposition products (Mg
2+ ions, hydroxides, oxides, chlorides, Mg-containing hydroxyapatite, hydrogen gas, and OH-) and they are generally not regulated by systemic renal control [
5]. These products can positively affect tissue metabolism reasonably, but a burst and intense release can represent an issue.
Several solutions have been proposed in the scientific literature to overcome the problem of too-fast degradation. While heat treatments, micro-alloying, and plastic deformation modify the alloy microstructure and, consequently, its mechanical properties [
6], surface modifications, and coatings seem the most promising strategies because they affect only surface properties and do not alter the mechanical ones. Among surface modifications, conversion and deposition coatings are the most studied. Conversion coatings are in situ grown coatings, which derive from specific reactions (chemical reactions of dissolution/deposition, anodization, plasma electrolytic oxidation (PEO), and alkali-heat treatments) on the material surface and the consequent development of a coating. Phosphates and oxides are the most diffused conversion coatings on Mg alloys [
6,
7]. Deposition of the coatings can be obtained using physical vapor deposition (PVD), atomic layer deposition (ALD), electrolytic deposition, sol–gel, and dip coating. Both inorganic (e.g., hydroxyapatite, diamond-like carbon, SiO
2, and TiO
2) and organic (polylactic acid—PLA, polylactic-co-glycolic acid—PLGA, polycaprolactone—PLC, and stearic acid—SA) coatings have been proposed for the degradation control of magnesium and its alloys [
6,
7]. Multifunctional properties of these coatings (e.g., osteointegration or drug loading abilities) can increase their potential, but a coating suitable to meet the medical needs is still far to be developed.
Polyphenols are natural molecules of vegetal origin widely studied for their numerous beneficial properties, such as the antioxidant, anti-inflammatory, antibacterial, anticancer, bone stimulating, and vasculoprotective ones, and are gaining increasing interest in industrial and medical applications [
8,
9]. Moreover, the corrosion protection abilities of polyphenols on metallic surfaces have been evidenced [
10,
11].
The possibility of exploiting the corrosion protection properties of polyphenols in the degradation control of magnesium alloys was recently explored in the scientific literature. Some works report the use of tannic acid [
12,
13], epigallocatechin gallate [
14,
15,
16], and gallic acid [
16,
17] for the obtainment of organic coatings on magnesium alloys for degradation control. Polyphenols were dissolved in water, and the majority of the coatings were produced by dip coating with one [
13,
16,
18] or more immersions (layer-by-layer approach) [
12,
14,
15] in the polyphenolic solution. A pre-oxidation treatment using immersion in NaOH [
11] or micro-arc oxidation (MAO) [
16] has been proposed in some cases to improve the substrate affinity for grafting. Moreover, some authors suggested the use of hexamethylenediamine [
17] or polyethyleneimine (PEI) [
15] to improve the bonding ability and stability of the coating, but the biocompatibility of these molecules is low. Finally, some authors [
12,
14] suggest the addition of magnesium ions to the solution of polyphenols for forming metal-phenol complexes useful for coating formation and adhesion.
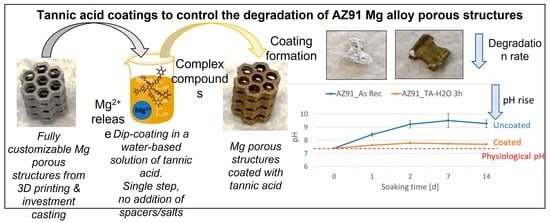
Three-dimensional porous structures have been selected because of their interesting clinical applications and because they maximize the degradation issue due to the large-exposed surface area. The hereby proposed cellular structures, cast from magnesium alloy, possessed orifices aiming the intensified tissue ingrowth. The honeycomb structure is light, stiff, and can be easily tailored. The foreseen application is focused on customized biodegradable magnesium-based implants (such as bone substitutes) with mechanical properties comparable to the natural bone that will be characterized by spatial structures beneficial for osseointegration and covered with protective coatings enhancing corrosion resistance and prolonging the bio-resorption time. Moreover, the production process of these structures (combination of 3D printing–fused deposition modeling, FDM, and investment casting) constitutes an innovative process. A certain innovation ensured by such an approach relates to the ease of customization of the implant’s shape. The technology is very flexible; nearly any kind of polymer pattern can be produced and replaced precisely by a chosen metal. In addition, the FDM method for pattern creation results in layered texture on the element’s surface, which is then transferred to the metal part. This effect may positively affect the tissue ingrowth and adhesion at the interface of magnesium casting and the deposited coating.
In the present research work, tannic acid coatings on AZ91 Mg alloy-porous structures were obtained from water-based solutions (water or phosphate-buffered saline—PBS) in a single passage without the addition of any spacer, salt, or toxic reagent. Moreover, the coating was obtained on as-prepared (only washed) substrates without pre-oxidation treatment. Compared to the strategies proposed in the literature, the method presented here is faster (single passage, no pre-treatment of the substrates), more sustainable (no employment of toxic substances), and cheaper (reduction of process time and reagents consumption). Furthermore, tannic acid–Mg complexes were formed by exploiting the magnesium release in the coating medium without adding magnesium salts. Lastly, acid tannic is known for its anti-bacterial and antioxidant action, and this coating has potential multifunctional abilities. Surfaces were characterized using scanning electron microscopy equipped with energy dispersive spectroscopy (SEM-EDS), Fourier transformed infrared spectroscopy (FTIR), tape adhesion test, fluorescence microscopy, Folin–Ciocalteu redox activity test, and degradation tests in PBS.
3. Results and Discussion
The compression strength was measured to ensure the repeatability of the mechanical properties of the 3D porous structures. AZ91 honeycombs, with Φ 1.5 mm orifices and cells of 3.5 mm, exhibited twofold higher strength during a quasi-static test, expressed by the plateau range in the stress–strain curve than observed for the other ones (orifices—Φ 3 mm, cells—6 mm). The forces recorded during compression at this stage were approximately 4 and 2 kN, respectively.
The tannic acid coating visually alters the color of the AZ91 surfaces, which move from bright grey to brownish, as shown in
Figure 1c,
Figure 2 and
Figure 3 for the 3D construct and small samples (prepared in different conditions of coating), respectively. However, no weight loss was noticed during the coating procedure, suggesting no alteration of the alloy during the process.
Figure 2 and
Figure 3 show the surface morphology of AZ91 samples before and after the TA coating. It can be observed that the grooved morphology (characteristic of the as-received samples) is maintained after the coating in all conditions. Such developed texture with an enlarged specific surface area is a direct result of the chosen manufacturing technology—deposition of PLA material in a layer-by-layer way during 3D printing. It may be beneficial for the ingrowth of biological tissue. This observation suggests the presence of a thin coating able to preserve the starting surface micro-texture and that no significant surface degradation/corrosion occurs during the coating process. The maintenance of the surface topography suggests a submicrometric thickness of the tannic acid coating.
The micro-scale appearance of the samples coated with tannic acid in water evidences a smoother morphology compared to the ones coated in PBS, suggesting the presence of a more homogeneous surface layer, as confirmed by EDS analyses in the following. Moreover, small precipitates and flakes are visible on the coatings obtained in water TA solution for over 30 min. They can be attributed to a more pronounced reaction of the metal in the coating solution and a greater presence of tannic acid on the surface.
Figure 4 plots the carbon content on the surface of the as-received and TA-coated, in different conditions, AZ91 samples. The TA coating induces an increase in the carbon content (from 7% to 37% for the TA coating formed in water for 3 h), which is significant, even if carbon is ubiquitous on surfaces and its quantification by EDS can be affected by instrumental errors up to 1%. The reported values cannot be quantitatively precise because of the limits of EDS in measuring the carbon content. Still, they suggest that the coating is more effective and homogeneous when performed in water than in PBS: higher carbon content (the carbon content rises to 37% when the coating is performed in water and only up to 12% when performed in PBS), lower standard deviation, and better substrate coverage (the magnesium content is reduced to 11% on samples coated in water and 22% on the ones coated in PBS from a starting content of 52%). In agreement with SEM observations, EDS results suggest a submicrometric coating thickness: the EDS penetration depth is close to 1 µm, and the Mg substrate is still detectable after the coating. The higher ability of TA to form a coating on Mg alloy when dissolved in water can be explained by considering the pH of the two solutions: it is 3.5 for the water solution and 6.1 for the PBS one. It can be hypothesized that more Mg
2+ ions are released into the solution in an acidic environment during the coating procedure. They act as bridges for the formation of tannic acid–magnesium ions complexes at the surface, as reported in the literature [
12,
14]. The authors have previously observed a similar effect for the Ca
2+ ions and polyphenols grafting on metallic surfaces [
22]. Moreover, a shielding effect due to the possible precipitation of magnesium phosphates from PBS [
1] cannot be excluded.
The EDS analyses on some exemplifying internal surfaces of a structure (denoted as IC, Internal Central, and I, Internal, as shown in
Figure 1d) coated in a water tannic acid solution for 3 h evidence a carbon content close to the one observed on the plane samples (30.8% for IC surface and 30.5% for the I surface), suggesting a homogeneous coating of the whole 3D surface, as also supported by the uniform color.
FTIR-ATR spectra of bare and coated samples are reported in
Figure 5, compared to the spectrum of tannic acid (TA).
As expected, no significant FTIR peaks can be noticed on bare AZ91 samples. On the other hand, the peaks, characteristic of tannic acid, clearly appear on the coated samples: OH phenolic group (a broad band around 3300 cm
−1), C = O (around 1700 cm
−1), the vibrations of the benzene ring (1700–1400 cm
−1), the vibration of carboxylic (1400–1200 cm
−1) and C-O groups (1200–1000 cm
−1) [
25,
26,
27]. FTIR confirms the presence of a coating of tannic acid.
The results of the tape adhesion test are reported in
Figure 6; a sample coated for 3 h in a tannic acid water solution was selected and tested.
The tape removes part of the coating during the test. However, a certain portion remains on the magnesium surface, as evidenced by the brownish areas on the sample after the test. The FTIR spectrum of the sample after the tape test (
Figure 5) confirms the permanence of a significant amount of tannic acid on the sample surface after the test. Considering that this specific coating is on bioresorbable implants, the need for good mechanical adhesion is mainly restricted to the surgery procedure. In future works, both a specific investigation of the adhesion during simulated implantation and the eventual improvement through a surface pre-treatment will be investigated by the authors.
Fluorescence microscopy (
Figure 7) evidences a complete absence of a fluorescent signal on the as-received material, as expected, and the presence of a visible red–orange signal on the coated AZ91 samples, which is particularly evident for the ones coated in the solution of tannic acid in water with an immersion time of 3 h. This effect is due to the polyphenols’ auto-fluorescence.
Looking at these results, the selected experimental condition for further experiments is a solution of tannic acid in water and an immersion time of 3 h for forming the coating.
The Folin–Ciocalteu test evidenced a concentration of polyphenols of 0.064 ± 0.003 mg/ml in gallic acid equivalent for the AZ91 samples coated with tannic acid using dipping for 3 h in a TA solution in water. The result indicates the presence of a significant amount of active tannic acid on the surface of the material, which is more than 1 order of magnitude higher than the one registered on titanium functionalized with natural polyphenols where the presence and activity were evidenced as significant for a biological response [
22]. The acid tannic coating’s potential antioxidant and anti-inflammatory action here finds first evidence.
Small samples, cut from the AZ91 3D structures, as-received and coated with tannic acid for 3 h in a water solution, were subjected to degradation tests. Each sample was soaked in PBS for up to 14 days and characterized for macro and micro appearances and chemical composition. In addition, the pH and magnesium content of the soaking solutions were measured at different experimental times. The results are reported in
Figure 8.
It can be observed that the visual appearance of the as-received samples significantly changes after soaking, especially from 7d on, with the reduction of the sample size and the formation of white deposits (
Figure 8a). On the other hand, the coated samples are almost unchanged even after 14 days of soaking in PBS. These results are confirmed by weight variations measurements which underline almost unchanged weight for TA coated samples (0.03%, −3.03%, and 0.43% weight variation at 1, 2, and 14 days, respectively) and a more pronounced and variable trend for uncoated ones (−2.73%, −1.04%, and 13.01% weight variation at 1, 2, and 14 days, respectively). Microscopic observations at SEM (
Figure 8a) confirm this result, showing a topography close to the one observable in
Figure 2 for the coated samples and the appearance of an increasing amount of deposits covering the pristine topography on the as-received ones.
The EDS analyses (
Figure 8b) confirm that the surface composition of the coated samples is almost unchanged. At the same time, a significant decrease in the magnesium content and an increase in oxygen can be noticed on the as-received samples after the degradation test. Even if not quantitatively precise because of the limits of EDS, carbon’s detected values align with a progressive and incomplete coating release during soaking. The permanence of the tannic coating, even after a prolonged soaking in PBS, is of great interest for an enhanced biological response.
The pH of PBS used for the degradation tests (
Figure 8c) maintains close to the physiological one in the case of the coated samples, while it rises to 8.5 after 1 day and up to 9.5 after 7 days in the case of the as-received samples.
Finally, a particular release of Mg
2+ ions in PBS can be observed during the degradation test (
Figure 8d), and it is significantly higher for the uncoated samples.
All these results suggest that the TA coating obtained by dipping the Mg alloy into a water solution can slow down the degradation of the AZ91 alloy in physiological conditions (PBS solution) and it is at least partially maintained on the surface.
The results show that the tannic acid coating acts as a barrier against dissolution since it homogeneously covers the magnesium alloy surface, protecting it from reacting with the surrounding liquid medium. Comparing the results obtained here with the literature, the efficacy of this simple process without any potentially toxic linker (such as hexamethylenediamine [
17]), salt (such as magnesium chloride or sulfate or hexavalent chromium compounds [
12,
28]), or expensive pre-treatment (such as micro-arc oxidation [
16] or multistep treatments to avoid the fast reaction between the reagents [
15]) was here proven for the AZ91 alloy. The obtained value of Mg
2+ ion release at 14 days in PBS is in line (about 40% less than the untreated alloy) with what is reported in the literature for similar coatings obtained by using hexamethylenediamine and gallic acid [
17] or tannic acid and magnesium chloride (with a pre-treatment in NaOH) [
12]. Furthermore, the pH values of PBS registered after a prolonged soaking of the coated samples are in line, or even lower, compared to those of pre-oxidized samples coated with gallic acid [
16]. What is relevant is that a 3D microporous structure was studied in the present paper with an enlarged effective area compared to a bulk sample. The investigated coating here is as effective as more complex ones on bulk samples with a lower surface area. A significant advantage of this process is also no risk of toxicity because of the exposition of amine groups [
15,
28].
In vivo tests show that it is not easy to realistically mimic the biological degradation process of magnesium alloys by immersion or electrochemical tests [
17] because of the difference due, for instance, to the encapsulating granulation tissue, which avoids magnesium hydroxide from falling from the substrate. On the other side, the results of in vitro degradation tests must be considered as preliminary values for a first screening of the coatings before in vitro biological tests or in vivo experiments without the ambition to measure the effective corrosion rate of an implant. The persistence of the coating after soaking in PBS for several days is of great interest considering the proven efficacy of polyphenols for an antioxidative (redox reactivity and scavengers of radical oxygen species) and anti-inflammatory action, as well as a selective action on different cell lines (suppression of fibroblast and smooth muscle cells growth and stimulation of osteoblastic cell proliferation) [
16].