Effect of Sb Addition on the Solidification of Deeply Undercooled Ag-28.1 wt. % Cu Eutectic Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Thermal History Analysis
3.2. DSC Analysis
3.3. XRD Analysis
3.4. Microstructures
3.5. Recalescence Rate
4. Discussion
4.1. Effect of Sb Addition on the Crystal Growth and the Formation of Anomalous Eutectic
4.2. Effect of Sb Addition on the Formation of Lamellar Eutectics
5. Conclusions
- (1)
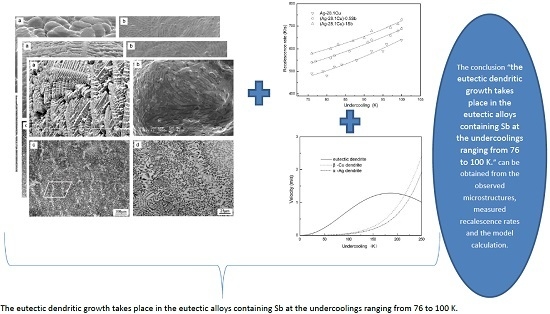
- The eutectic dendritic growth takes place in the eutectic alloys containing Sb at the undercoolings ranging from 76 to 100 K. The remelting and ripening of the original lamellar eutectics result in the formation of anomalous eutectics.
- (2)
- For a fixed undercooling, the recalescence rate and the volume fraction of anomalous eutectics increase with the increase of Sb content. For a fixed Sb content, the recalescence rate increases with the increase of undercooling, and there is a jump at 76 K, indicating that the developed eutectic dendrites grow much faster.
- (3)
- The additional constitutional supercooling in the liquid ahead of the eutectic interface caused by Sb addition results in the dendritic growth of lamellar eutectics outward from the anomalous eutectics at the stage of slow solidification of the eutectic alloys containing Sb.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, M.F.; Hong, C.P. Modeling of microstructure evolution in regular eutectic growth. Phys. Rev. B 2002, 66, 1554281–1554288. [Google Scholar] [CrossRef]
- Folch, R.; Plapp, M. Towards a quantitative phase-field model of two-phase solidification. Phys. Rev. E 2003, 68, 106021–106024. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Liu, F.; Xu, X.L.; Dang, B. Undercooled solidification of Ni-3.3 wt. % B alloy and cooling curve description. Mater. Sci. Technol. 2013, 29, 36–42. [Google Scholar] [CrossRef]
- Han, X.J.; Wei, B. Microstructural characteristics of Ni-Sb eutectic alloys under substantial undercooling and containerless solidification conditions. Metall. Mater. Trans. A 2002, 33, 1221–1228. [Google Scholar] [CrossRef]
- Yang, C.; Gao, J.; Zhang, Y.K.; Kolbe, M.; Herlach, D.M. New evidence for the dual origin of anomalous eutectic structures in undercooled Ni-Sn alloys: In situ observations and EBSD characterization. Acta Mater. 2011, 59, 3915–3926. [Google Scholar] [CrossRef]
- Liu, L.; Wei, X.X.; Huang, Q.S.; Li, J.F.; Cheng, X.H.; Zhou, Y.H. Anomalous eutectic formation in the solidification of undercooled Co-Sn alloys. J. Cryst. Growth 2012, 358, 20–28. [Google Scholar] [CrossRef]
- Clopet, C.R.; Cochrane, R.F.; Mullis, A.M. The origin of anomalous eutectic structures in undercooled Ag-Cu alloy. Acta Mater. 2013, 61, 6894–6902. [Google Scholar] [CrossRef]
- Wei, X.X.; Lin, X.; Xu, W.; Huang, Q.S.; Ferry, M.; Li, J.F.; Zhou, Y.H. Remelting-induced anomalous eutectic formation during solidification of deeply undercooled eutectic alloy melts. Acta Mater. 2015, 95, 44–56. [Google Scholar] [CrossRef]
- Li, M.; Nagashio, K.; Ishikawa, T.; Yoda, S.; Kuribayashi, K. Microtexture and macrotexture formation in the containerless solidification of undercooled Ni-18.7 at. % Sn eutectic melts. Acta Mater. 2005, 53, 731–741. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Zhang, D. In situ observation of solidification of undercooled hypoeutectic Ni-Ni3B alloy melt. J. Mater. Res. 2013, 28, 1891–1902. [Google Scholar] [CrossRef]
- Mullis, A.M. The origins of spontaneous grain refinement in deeply undercooled metallic melts. Metals 2014, 4, 155–167. [Google Scholar] [CrossRef]
- Herlach, D.M.; Feuerbacher, B. Non-equilibrium solidification of undercooled metallic melts. Metals 2014, 4, 196–234. [Google Scholar] [CrossRef]
- Liu, N.; Yang, G.C.; Liu, F.; Chen, Y.Z.; Yang, C.L.; Lu, Y.P. Grain refinement and grain coarsening of undercooled Fe-Co alloy. Mater. Charact. 2006, 57, 115–120. [Google Scholar] [CrossRef]
- Wei, B.; Yang, G.C.; Zhou, Y.H. High undercooling and rapid solidification of Ni-32.5% Sn eutectic alloy. Acta Metall. Mater. 1991, 39, 1249–1258. [Google Scholar]
- Subramanian, P.R.; Perepezko, J.H. The Ag-Cu (Silver-Copper) system. J. Phase Equilibria 1993, 14, 62–75. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.F.; Liu, L.; Zhou, Y.H. Solidification of undercooled Ag-Cu eutectic alloy with the Sb addition. J. Alloy. Compd. 2009, 478, 252–256. [Google Scholar] [CrossRef]
- Goetzinger, R.; Barth, M.; Herlach, D.M. Mechanism of formation of the anomalous eutectic structure in rapidly solidified Ni-Si, Co-Sb and Ni-Al-Ti alloys. Acta Mater. 1998, 46, 1647–1655. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Coriell, S.R.; Trivedi, R. Principles and technologies. In Rapid Solidification Processing; Mehrabian, R., Parrish, P.A., Eds.; Claitors Publications: Baton Rouge, LA, USA, 1988; p. 13. [Google Scholar]
- Zhao, S.; Li, J.F.; Liu, L.; Zhou, Y.H. Effect of solute trapping on the growth process in undercooled eutectic melts. Acta Metall. Sin. 2008, 44, 1335–1339. [Google Scholar]
- Li, J.F.; Zhou, Y.H. Eutectic growth in bulk undercooled melts. Acta Mater. 2005, 53, 2351–2359. [Google Scholar] [CrossRef]
- Walder, S.; Ryder, P.L. Critical solidification behavior of undercooled Ag-Cu alloys. J. Appl. Phys. 1993, 74, 6100–6106. [Google Scholar] [CrossRef]
- Wang, N.; Cao, C.D.; Wei, B.B. Rapid solidification of Ag-Cu eutectic alloy by drop tube processing. Acta Metall. Sin. 1998, 34, 824–830. [Google Scholar]
- Li, J.F.; Li, X.L.; Liu, L.; Lu, S.Y. Mechanism of anomalous eutectic formation in the solidification of undercooled Ni-Sn eutectic alloy. J. Mater. Res. 2008, 23, 2139–2148. [Google Scholar] [CrossRef]
- Liu, X.R.; Cao, C.D.; Wei, B. Microstructure evolution and solidification kinetics of undercooled Co-Ge eutectic alloys. Scr. Mater. 2002, 46, 13–18. [Google Scholar] [CrossRef]
- Yao, W.J.; Han, X.J.; Wei, B. Microstructural evolution during containerless rapid solidification of Ni-Mo eutectic alloys. J. Alloy. Compd. 2003, 348, 88–99. [Google Scholar] [CrossRef]
- Aziz, M.J. Model for solute redistribution during rapid solidification. J. Appl. Phys. 1982, 53, 1158–1168. [Google Scholar] [CrossRef]
- Goetzinger, R.; Barth, M.; Herlach, D.M. Growth of lamellar eutectic dendrites in undercooled melts. J. Appl. Phys. 1998, 84, 1643–1649. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 4th ed.; Trans Tech Publications Ltd.: Dürnten, Switzerland, 1998; p. 108. [Google Scholar]









| Parameter | Value |
|---|---|
| Latent heat of fusion for α-Ag/β-Cu (J/mol) | 11,326/13,027 |
| Specific heat of the liquid (J/mol·K) | 32.21 |
| Interdiffusion coefficient of solutes (m2/s) | 2.42 × 10−7exp(−48,886/8.314T) |
| Volume fraction of α-Ag in the eutectic | 0.74 |
| Thermal diffusivity in the liquid (m2/s) | 5.44 × 10−5 |
| Gibbs-Thomson parameter for α-Ag/β-Cu (m·K) | 1.5 × 10−7/1.3 × 10−7 |
| Eutectic temperature (K) | 1053 |
| Liquidus slope for α-Ag/β-Cu (K/at. %) | −4.56/5.07 |
| Kinetic parameter for α-Ag/β-Cu (m/s·K) | 0.61/0.71 |
| Equilibrium solute partition coefficient | 0.353 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Chen, Y.; Wei, D. Effect of Sb Addition on the Solidification of Deeply Undercooled Ag-28.1 wt. % Cu Eutectic Alloy. Metals 2016, 6, 39. https://doi.org/10.3390/met6020039
Zhao S, Chen Y, Wei D. Effect of Sb Addition on the Solidification of Deeply Undercooled Ag-28.1 wt. % Cu Eutectic Alloy. Metals. 2016; 6(2):39. https://doi.org/10.3390/met6020039
Chicago/Turabian StyleZhao, Su, Yunxia Chen, and Donglai Wei. 2016. "Effect of Sb Addition on the Solidification of Deeply Undercooled Ag-28.1 wt. % Cu Eutectic Alloy" Metals 6, no. 2: 39. https://doi.org/10.3390/met6020039
APA StyleZhao, S., Chen, Y., & Wei, D. (2016). Effect of Sb Addition on the Solidification of Deeply Undercooled Ag-28.1 wt. % Cu Eutectic Alloy. Metals, 6(2), 39. https://doi.org/10.3390/met6020039