3. Results and Discussion
Figure 1 shows OM/EDS/XRD results of the Al hot-dipped carbon steel.
Figure 1a indicates that the hot-dipped coating consists of the Al topcoat with a thickness of about 50–60 µm and the Al-Fe alloy layer with a thickness of 170–230 µm. The characteristic finger- or tongue-like morphology was seen between the Al-Fe alloy layer with the columnar microstructure and the steel substrate having equiaxed grains [
8]. It originated from the fast inward diffusion of Al during hot-dipping through the orthorhombic Al
5Fe
2 with 30% vacancies along the
c-axis [
4], or from the atomic size mismatch between Al (atomic radius; 0.143 nm) and Fe (atomic radius; 0.126 nm) [
9].
Figure 1b indicates the EDS concentration spot analysis (at. %). Spots 1–4, 5–19 and 20–22 corresponded to the Al topcoat, Al-Fe alloy layer, and the steel substrate, respectively. The Al/Fe compositional ratios (at. %) at spots 5–6 and 7–19 were 2.9 and 2.6, respectively, suggesting that mainly Al
3Fe and Al
5Fe
2 were formed in the alloy layer between the topcoat and the substrate. SEM/EDS analysis indicated that no aluminum was detected below spot 19. The alloying elements such as Mn and Si were detected in a small amount inside the coating (
Figure 1b), implying their dissolution in the coating. The intensity of diffraction patterns decreased in the order of Al, Al
5Fe
2, and Al
3Fe in
Figure 1c, and decreased in the order of Al
5Fe
2 and Al
3Fe in
Figure 1d. This indicated that the Al-Fe alloy layer that existed below the Al topcoat was composed of Al
5Fe
2 as the major phase, and Al
3Fe as the minor one [
3,
4].
Figure 2 shows XRD/OM results of the Al hot-dipped carbon steel after corrosion at 600 °C for 50 h. In
Figure 2a, α-Al
2O
3 and Al
5Fe
2 were detected as the minor and major phases, respectively. The Al topcoat partially oxidized to α-Al
2O
3, and leftover (
i.e., uncorroded) aluminum topcoat along with the underlying Al
3Fe layer transformed to the Al
5Fe
2 not only due to the outward diffusion of Fe (from the substrate to the surface) but also due to the inward diffusion of Al (from the coating towards the interior) during heating under N
2/0.1% H
2S gas. The oxygen source for the formation of α-Al
2O
3 was the impurity of oxygen in the N
2/0.1% H
2S gas. The α-Al
2O
3 scale with a thickness of about 5 µm, the underlying Al
5Fe
2-rich alloy layer, and the substrate were shown in
Figure 2b. The α-Al
2O
3 scale was non-adherent due to the incorporation of sulfur and hydrogen from H
2S and the mismatch of thermal expansion coefficients between the scale and the alloy layer.
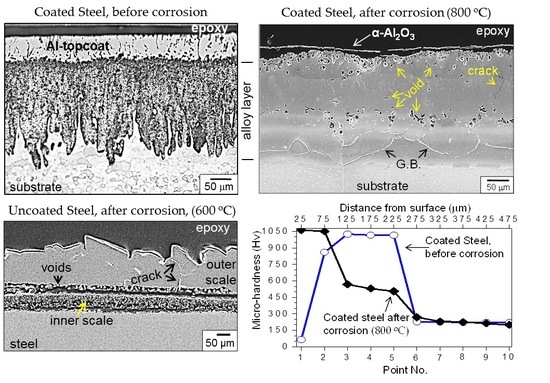
Without aluminum hot-dipping, the carbon steel was corroded seriously, as shown in
Figure 3. The corrosion condition selected for the uncoated steel was the same as described in
Figure 2 (
i.e., 600 °C for 50 h) in order to find the effect of hot-dip aluminizing. The scale formed on the uncoated steel was ~165–200 µm thick (
Figure 3a), and consisted of FeS (
Figure 3b). The scale was fragile and easily detached from the matrix owing to the incorporation of sulfur and hydrogen from H
2S. Moreover, excessively large growth stress had arisen due to the formation of a thick scale, and the thermal stresses generated during corrosion and the subsequent cooling stage. Since FeS has a defect structure of Fe
1−xS (
x = 0–0.2), it grows rapidly through the outward diffusion of Fe
2+ ions, leaving Kirkendall voids behind [
1]. Hence, numerous voids formed at the bottom of the FeS scale, which could act as stress concentration sites to facilitate the scale spallation (
Figure 3a).
Figure 4 shows SEM/EDS/XRD results of the Al hot-dipped carbon steel after corrosion at 800 °C for 100 h. In
Figure 4a, the α-Al
2O
3 scale was about 5 µm thick, non-adherent, and partially broken owing to the excessive stress accumulated in the scale as well as the hydrogen and sulfur released from the H
2S gas. According to the SEM-EDS analysis, the composition of spot 1 shown in
Figure 4b was 38.9% Al-58.1% O-2.8% S-0.2% Fe in wt. %, which indicated that α-Al
2O
3 was incorporated with some sulfur and iron. Such was also observed at the spot 2 shown in
Figure 4b. Sulfur and iron ions that were doped in the highly stoichiometric α-Al
2O
3 may constitute additional energy levels localized in the forbidden energy band, which would beneficially increase the defect mobility in α-Al
2O
3 and thereby enhance the formation of α-Al
2O
3. Sulfur was present only at spot 1 (2.8 wt. %) and spot 2 (1.6 wt. %). However, oxygen was present all over spots 1–25, with its concentration (wt. %) gradually decreased from 58.1% O at spot 1 to 0.6% O at spot 25. This suggested that oxygen diffused inwardly faster and more in-depth than sulfur. Nevertheless, the thin α-Al
2O
3 scale effectively protected the steel, suppressing the formation of the non-protective FeS or less protective Al
2S
3 scale at the coating surface. In
Figure 4a, spots 1–15 were not able to be etched due to the dissolution of oxygen and sulfur to a certain amount, while spots 15–25 were able to be etched, which revealed coarse, fully grown substrate grains. Hence, the boundary between the retained coating and the substrate may be designated as spot 15. Numerous voids formed at spots 12–16, and furthermore, at spots 2–4 due to the interdiffusion between the coating and the substrate. The amount of void formation increased with an increase in the corrosion temperature and time. A few cracks formed due to thermal expansion mismatch between coating and the steel substrate [
6]. Because of the counter-diffusion of Al and Fe, the characteristic finger- or tongue-like morphology as shown in
Figure 1a was no longer observable in
Figure 4a. In
Figure 4c, the strongest peaks were in the order of Al
13Fe
4 and AlFe
3, indicating that the upper part of the retained coating (
viz around spot 3 in
Figure 4a) consisted of Al
13Fe
4 as the major phase and AlFe
3 as the minor phase. Additionally, faint peaks of α-Al
2O
3 and FeS were detected in
Figure 4c. A small amount of FeS was formed at the coating surface not only because of the α-Al
2O
3 scale spallation but also due to the preferential oxidation of Al at the outer surface. When the upper part of the coating was ground off to reveal the underlying phases, Al
13Fe
4 became the major phase (
Figure 4d). The EDS concentration profile also corresponded to the Al
13Fe
4 phase around the ground off area (
viz spots 19 and 20). The alloying elements such as Mn and Si tended to be present more around spots 21–25, apparently owing to the inward transportation of Al that expelled Mn and Si.
Figure 5 shows the microhardness profile of the Al hot-dipped carbon steel. Before heating, the microhardness (Hv) was about 65 for point 1 (
viz Al topcoat), 859 for point 2 (
viz Al
3Fe), 1020 for points 3–5 (
viz Al
5Fe
2 in the alloy layer), and 223 for points 6–10 (
viz α-Fe substrate). Such hardness increment due to hot-dipping can improve the wear resistance and beneficially enhance the service life of the steel [
6]. After heating at 800 °C for 100 h, the microhardness of points 1 and 2 increased mainly due to oxidation or the incorporation of oxygen in the coating, whereas that of points 3–6 decreased mainly due to the counter-diffusion of Al and Fe, which changed the brittle Al
5Fe
2-rich alloy layer to the ductile (AlFe or Al
13Fe
4)-rich alloy layer or another ductile Al-dissolved layer. Ductile AlFe and Al
13Fe
4 may be used as structural components because of their good oxidation- and corrosion-resistance, and high specific strength [
5]. The microhardness of the substrate did not change noticeably before and after heating.
Figure 6 shows the Ellingham diagram (data from [
10]). The oxides were more stable than the corresponding sulfides, whereas Al was more active than Fe. Hence, the most stable α-Al
2O
3 formed preferentially upon exposure of the Al hot-dipped steel to N
2/0.1% H
2S mixed gas, and suppressed the corrosion significantly. By contrast, the uncoated steel degraded rapidly not by oxidation but by sulfidation. Upon exposure of the uncoated steel to N
2/0.1% H
2S mixed gas, FeO and FeS would form competitively. Since FeS is much more non-stoichiometric than FeO, FeS overgrows FeO and covers the whole surface as the corrosion proceeds. The fast growth rate of FeS, incorporation of hydrogen and sulfur, void formation, and excessive stress that generated owing to the compositional difference in the scale resulted in the cracking and detachment of the FeS scale, allowing the direct contact of N
2/0.1% H
2S gas with the uncoated steel. This further accelerated the corrosion of the uncoated steel.