Effect of Synthesizing Temperature on Microstructure and Electrochemical Property of the Hydrothermal Conversion Coating on Mg-2Zn-0.5Mn-Ca-Ce Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesizing Process of Conversion Coating
2.2. Microstructure Characterization
2.3. Corrosion Tests
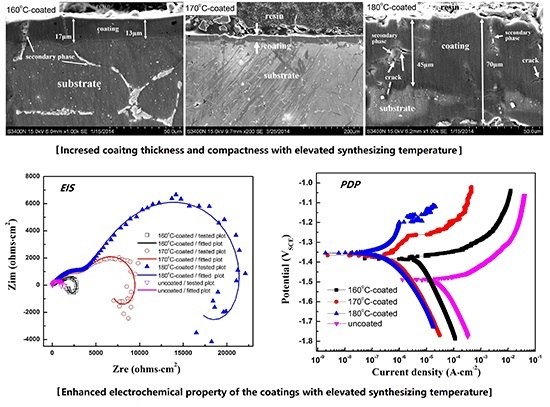
3. Results and Discussion
3.1. Microstructure Characteristic of the Coatings
3.2. Electrochemical Properties of the Coatings
3.3. Effect of Synthesizing Temperaturse on the Coating Formation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—applications—potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, B.P.; Wang, Y.; Geng, L.; Jiao, X.H. Preparation and characterization of a new biomedical Mg-Zn-Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shen, T.; Aung, N.N. Effect of heat treatment on corrosion behaviour of magnesium alloy AZ91D in simulated body fluid. Corros. Sci. 2010, 52, 1035–1041. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chen, M.S.; Lin, L.H.; Lin, M.H.; Wu, C.Z.; Ou, K.L.; Yu, C.H. Effect of heat treatment on the microstructures and damping properties of biomedical Mg-Zr alloy. J. Alloy. Compd. 2011, 509, 813–819. [Google Scholar] [CrossRef]
- Cui, X.J.; Lin, X.Z.; Liu, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coatingon AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Atrens, A.; Cao, F.; Shi, Z.; Dargusch, M.S. 3-Corrosion of Mg for Biomedical Applications. In Surface Coating Modification of Metallic Biomaterials; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–102. [Google Scholar]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F.; Zhang, Y.Q.; Zhang, S.F.; Qu, B.; Guo, S.B.; Xiang, J.H. Formation process of micro arc oxidation coatings obtained in a sodium phytate containing solution with and without CaCO3 on binary Mg-1.0Ca alloy. Appl. Surf. Sci. 2015, 325, 79–85. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Beni, B.H.; Vashaee, D.; Tayebi, L. Nanostructured merwinite bioceramic coating on Mg alloy deposited by electrophoretic deposition. Ceram. Int. 2014, 40, 9473–9484. [Google Scholar] [CrossRef]
- Jiang, X.J.; Guo, R.G.; Jiang, S.Q. Microstructure and corrosion resistance of Ce-V conversion coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2015, 341, 166–174. [Google Scholar] [CrossRef]
- Lu, Y.J.; Wan, P.; Tan, L.L.; Zhang, B.C.; Yang, K.; Lin, J.X. Preliminary study on a bioactive Sr containing Ca-P coating on pure magnesium by a two-step procedure. Surf. Coat. Technol. 2014, 252, 79–86. [Google Scholar] [CrossRef]
- Jeong, H.; Yoo, Y. Synthesis and characterization of thin films on magnesium alloy using a hydrothermal method. Surf. Coat. Technol. 2015, 284, 26–30. [Google Scholar] [CrossRef]
- Tomozawa, M.; Hiromoto, S. Growth mechanism of hydroxyapatite-coatings formed on pure magnesium and corrosion behavior of the coated magnesium. Appl. Surf. Sci. 2011, 257, 8253–8257. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, A.B.; Song, D.; Jiang, J.H.; Lu, F.M.; Zhang, L.Y.; Yang, D.H.; Chen, J.Q. Improving in-vitro biocorrosion resistance of Mg-Zn-Mn-Ca alloy in Hanks’ solution through addition of cerium. J. Rare Earth 2015, 33, 93–101. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, G.; Zhang, Y.H.; Zhao, Q. Growth and characterization of Mg(OH)2 film on magnesium alloy AZ31. Appl. Surf. Sci. 2011, 257, 6129–6137. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mensah-Darkwa, K.; Kumar, D. Corrosion protective conversion coatings on magnesium disks using a hydrothermal technique. J. Mater. Sci. Technol. 2014, 30, 47–53. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhao, Q.; Zhang, Y.H.; Wu, G.M. Hydrothermal synthesis of protective coating on magnesium alloy using de-ionized water. Surf. Coat. Technol. 2012, 206, 2961–2966. [Google Scholar] [CrossRef]
- Zhou, W.; Shan, D.; Han, E.H.; Ke, W. Structure and formation mechanism of phosphate conversion coating on die-cast AZ91D magnesium alloy. Corros. Sci. 2008, 50, 329–337. [Google Scholar] [CrossRef]
- Mosiałek, M.; Mordarski, G.; Nowak, P.; Simka, W.; Nawrat, G.; Hanke, M.; Socha, R.P. Phosphate-permanganate conversion coatings on the AZ81 magnesium alloy: SEM, EIS and XPS studies. Surf. Coat. Technol. 2011, 206, 51–62. [Google Scholar] [CrossRef]
- Rzazvi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vitro study of nanostructured diopside coating on Mg alloy orthopedic implants. Mater. Sci. Eng. C 2014, 41, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y.; Wong, M.H.; Cheng, F.T.; Man, H.C. Characterization and corrosion studies of fluoride conversion coating on degradable Mg implants. Surf. Coat. Technol. 2007, 202, 590–598. [Google Scholar] [CrossRef]








| Zn | Mn | Ca | Ce | Mg |
|---|---|---|---|---|
| 2.00 | 0.50 | 1.02 | 1.35 | balance |
| Solution | Chemical Composition (mM) | |||||||
|---|---|---|---|---|---|---|---|---|
| NaCl | CaCl2 | MgSO4 | KCl | KH2PO4 | Na2HPO4 | d-Glucose | NaHCO3 | |
| Hanks’ | 137 | 1.261 | 0.814 | 5.33 | 0.44 | 0.338 | 5.56 | 4.17 |
| Sample | Rs (Ω·cm2) | Cf (10−6 F·cm−2) | Rp (Ω·cm2) | Cdl (10−6 F·cm−2) | Rt (Ω·cm2) |
|---|---|---|---|---|---|
| uncoated | 24.67 | / | / | 128.4 | 847 |
| 160 °C-coated | 14.96 | 31.1 | 1480 | 71.8 | 1204 |
| 170 °C-coated | 13.19 | 19.2 | 6000 | 37.5 | 2148 |
| 180 °C-coated | 10.36 | 12.8 | 14800 | 26.1 | 4830 |
| Samples | Ecorr (V) | Icorr (A·cm−2) |
|---|---|---|
| uncoated | −1.486 | 5.85 × 10−5 |
| 160 °C-coated | −1.383 | 5.42 × 10−6 |
| 170 °C-coated | −1.366 | 4.59 × 10−7 |
| 180 °C-coated | −1.354 | 2.78 × 10−7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Song, D.; Jiang, J.; Ma, A.; Zhang, L.; Li, C. Effect of Synthesizing Temperature on Microstructure and Electrochemical Property of the Hydrothermal Conversion Coating on Mg-2Zn-0.5Mn-Ca-Ce Alloy. Metals 2016, 6, 44. https://doi.org/10.3390/met6030044
Guo G, Song D, Jiang J, Ma A, Zhang L, Li C. Effect of Synthesizing Temperature on Microstructure and Electrochemical Property of the Hydrothermal Conversion Coating on Mg-2Zn-0.5Mn-Ca-Ce Alloy. Metals. 2016; 6(3):44. https://doi.org/10.3390/met6030044
Chicago/Turabian StyleGuo, Guanghui, Dan Song, Jinghua Jiang, Aibin Ma, Liwen Zhang, and Cheng Li. 2016. "Effect of Synthesizing Temperature on Microstructure and Electrochemical Property of the Hydrothermal Conversion Coating on Mg-2Zn-0.5Mn-Ca-Ce Alloy" Metals 6, no. 3: 44. https://doi.org/10.3390/met6030044
APA StyleGuo, G., Song, D., Jiang, J., Ma, A., Zhang, L., & Li, C. (2016). Effect of Synthesizing Temperature on Microstructure and Electrochemical Property of the Hydrothermal Conversion Coating on Mg-2Zn-0.5Mn-Ca-Ce Alloy. Metals, 6(3), 44. https://doi.org/10.3390/met6030044