Al-Co Alloys Prepared by Vacuum Arc Melting: Correlating Microstructure Evolution and Aqueous Corrosion Behavior with Co Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Fabrication
2.2. Microstructure, Composition and Hardness Evaluation
2.3. Corrosion Testing
3. Results and Discussion
3.1. Microstructural Analysis
3.1.1. Microstructure Evolution
3.1.2. Microstructure and Composition Data
3.2. Corrosion Performance in 3.5 wt.% NaCl
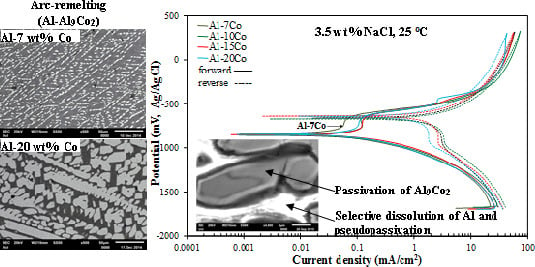
3.2.1. Polarization Behavior of Al-Co
3.2.2. Polarization Behavior of CP-Al
3.2.3. Comparison of the Polarization Behavior of the Various Compositions
3.2.4. Microstructure of Corrosion
3.2.5. Surface Film Identification
3.2.6. Mechanism of Corrosion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RS | Rapid solidification |
| MS | Melt spinning |
| CMA | Complex metallic alloys |
| TM | Transition metals |
| VAR | Vacuum arc remelting |
| CP | Commercially pure |
| SE | Secondary electron |
| BSE | Back scattered electron |
References
- Sater, J.M.; Jha, S.C.; Sanders, T.H., Jr. Microstructure and Properties of Rapidly Solidified Aluminum-Transition Metal Alloys in Aluminum Alloys. In Aluminum alloys-Contemporary Research and Applications, Treatise on Materials Science and Technology; Vasudevadan, A.K., Doherty, R.D., Eds.; Academic Press Inc.: San Diego, CA, USA, 1989; pp. 410–442. [Google Scholar]
- Karakose, E.; Keskin, M. Structural investigations of mechanical properties of Al based rapidly solidified alloys. Mater. Des. 2011, 32, 4970–4979. [Google Scholar] [CrossRef]
- Nayak, S.S.; Chang, H.J.; Kim, D.H.; Pabi, S.K.; Murty, B.S. Formation of metastable phases and nanocomposite structures in rapidly solidified Al-Fe alloys. Mater. Sci. Eng. A 2011, 528, 5967–5973. [Google Scholar] [CrossRef]
- Gogebakan, M.; Uzun, O.; Karaaslan, T.; Keskin, M. Rapidly solidified Al-6.5 wt.% Ni alloy. J. Mater. Process. Technol. 2003, 142, 87–92. [Google Scholar] [CrossRef]
- Katgerman, L.; Dom, F. Rapidly solidified aluminium alloys by melt spinning. Mater. Sci. Eng. A 2004, 375–377, 1212–1216. [Google Scholar] [CrossRef]
- Lufti Ovekoglu, M.; Unlu, N.; Eruslu, N.; Genc, A. Characterization investigations of a melt-spun ternary Al-8Si-5.1Cu (in wt.%) alloy. Mater. Lett. 2003, 57, 3296–3301. [Google Scholar] [CrossRef]
- Karakose, E.; Keskin, M. Microstructure and microhardness evolutions of melt-spun Al-8Ni-5Nd-4Si alloy. Mater. Character. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Vidoz, A.E.; Crooks, D.D.; Lewis, R.E.; Palmer, I.G.; Wadsworth, J. Ultralow-Density, High-Modulus, and High-Strength RSP Al-Li-Be Alloys. In Rapidly Solidified Powder Aluminum Alloys; Fine, M.E., Starke, E.A., Jr., Eds.; ASTM: Philadelphia, PA, USA, 1986; pp. 237–251. [Google Scholar]
- Yoshioka, H.; Yoshida, S.; Kawashima, A.; Asami, K.; Hashimoto, K. The pitting corrosion behavior of rapidly solidified aluminum alloys. Corros. Sci. 1986, 26, 795–812. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Liang, P.; Song, X.; Hu, Q.; Sun, Z.; Song, X.; Yang, S.; Wang, L. The electrochemical properties of melt-spun Al-Si-Cu alloys. Mater. Chem. Phys. 2011, 129, 1006–1010. [Google Scholar] [CrossRef]
- Menon, J.; Suryanarayana, C. Metallography of a melt-quenched aluminum-cobalt alloy. Metallography 1988, 21, 179–197. [Google Scholar] [CrossRef]
- Froes, F.H.; Kim, Y.W.; Murthy, S.K. Rapid solidification of lightweight metal alloys. Mater. Sci. Eng. A 1989, 117, 19–32. [Google Scholar] [CrossRef]
- Adam, A.M. Influence of Rapid Solidification on the Microstructure of Aluminium Rich Hypereutectic Al-Co Alloys; U.P.B. Sci. Bull. Ser. B; University Polytechnica of Bucharest: Bucharest, Romania, 2011; Volume 73, pp. 217–228. [Google Scholar]
- Garrett, R.K., Jr.; Sanders, T.H., Jr. The formation of coarse intermetallics in rapidly solidified Al-Co alloys. Mater. Sci. Eng. 1983, 60, 269–274. [Google Scholar] [CrossRef]
- Yamauchi, I.; Kawamura, H.; Nakano, K.; Tanaka, T. Formation of fine skeletal Co-Ag by chemical leaching of Al-Co-Ag ternary alloys. J. Alloys Compd. 2005, 287, 187–192. [Google Scholar] [CrossRef]
- Armbrüster, M.; Schlögl, R.; Grin, Y. Intermetallic compounds in heterogeneous catalysis—A quickly developing field. Sci. Technol. Adv. Mater. 2014, 15, 17. [Google Scholar] [CrossRef]
- Dubois, J.M.; Belin-Ferré, E.; Feuerbacher, M. Introduction to the Science of Complex Metallic Alloys. In Complex Metallic Alloys: Fundamentals and Applications; Dubois, J.M., Belin-Ferré, E., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2011; pp. 1–39. [Google Scholar]
- Urban, K.; Feuerbacher, M. Structurally complex alloy phases. J. Non-Cryst. Solids 2004, 334–335, 143–150. [Google Scholar] [CrossRef]
- Barthes-Labrousse, M.G.; Dubois, J.M. Quasicrystals and complex metallic alloys: Trends for potential applications. Philos. Mag. 2008, 88, 2217–2225. [Google Scholar] [CrossRef]
- Eckert, J.; Scudino, S.; Stoica, M.; Kenzari, S.; Sales, M. Mechanical Engineering Properties of CMAs. In Complex Metallic Alloys: Fundamentals and Applications; Dubois, J.M., Belin-Ferré, E., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2011; pp. 273–315. [Google Scholar]
- Alarcon Villaseca, S.; Ledieu, J.; Serkovic Loli, L.N.; De Weerd, M.C.; Gille, P.; Fournée, V.; Dubois, J.M.; Gaudry, E. Structural investigation of the (001) surface of the Al9Co2 Complex Metallic Alloy. J. Phys. Chem. 2011, C115, 14922–14932. [Google Scholar]
- Alarcon Villaseca, S.; Serkovic Loli, L.N.; Ledieu, J.; Fournée, V.; Gille, P.; Dubois, J.M.; Gaudry, E. Oxygen adsorption on the Al9Co2(001) surface: First principles and STM study. J. Phys. Condens. Matter. 2013, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, E.H.; Hunsicker, H.Y. Corrosion of Aluminium and Aluminium alloys. In ASM Handbook (Vol. 13): Corrosion, 9th ed.; Davis, J.R., Ed.; ASM International: Ohio, OH, USA, 1987; pp. 583–609. [Google Scholar]
- Lucente, A.M.; Scully, J.R. Pitting of Al-based amorphous nanocrystalline alloys with solute-lean nanocrystals. Electrochem. Solid-State Lett. 2007, 10, C39–C43. [Google Scholar] [CrossRef]
- Goldman, M.A.; Unlu, N.; Shiflet, G.J.; Scully, J.R. Selected corrosion properties of a novel amorphous Al-Co-Ce alloy system. Electrochem. Solid-State Lett. 2005, 8, B1–B5. [Google Scholar] [CrossRef]
- Presuel-Moreno, F.; Jakab, M.A.; Tailleart, N.; Goldman, M.; Scully, J.R. Corrosion resistant metallic coatings. Mater. Today 2008, 11, 14–23. [Google Scholar] [CrossRef]
- Palcut, M.; Priputen, P.; Kusy, M.; Janovec, J. Corrosion behaviour of Al-29 at.%Co in aqueous NaCl. Corros. Sci. 2013, 75, 461–466. [Google Scholar] [CrossRef]
- Palcut, M; Priputen, P.; Salgo, K.; Janovec, J. Phase constitution and corrosion resistance of Al-Co alloys. Mater. Chem. Phys. 2015, 166, 95–104. [Google Scholar]
- Lekatou, A.; Sfikas, A.K.; Karantzalis, A.E.; Sioulas, D. Microstructure and corrosion performance of Al-32% Co alloys. Corros. Sci. 2012, 63, 193–209. [Google Scholar] [CrossRef]
- Stoloff, N.; Liu, C.; Deevi, S. Emerging applications of intermetallics. Intermetallics 2000, 8, 1313–1320. [Google Scholar] [CrossRef]
- Principe, E.; Shaw, B.; Davis, G. Role of oxide/metal interface in corrosion resistance: Al-W and Al-Mo systems. Corrosion 2003, 59, 295–313. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A. Effect of chromium on the electrochemical properties of iron aluminide intermetallics. Corros. Sci. 2014, 78, 223–232. [Google Scholar] [CrossRef]
- Rajamure, R.S.; Vora, H.D.; Srinivasan, S.G.; Dahotre, N.D. Laser alloyed Al-W coatings on aluminum for enhanced corrosion resistance. App. Surf. Sci. 2015, 328, 205–214. [Google Scholar] [CrossRef]
- Ura-Binczyk, E.; Homazava, N.; Ulrich, A.; Hauert, R.; Lewandowska, M.; Kurzydlowski, K.J.; Schmutz, P. Passivation of Al–Cr–Fe and Al–Cu–Fe–Cr complex metallic alloys in 1 M H2SO4 and 1 M NaOH solutions. Corros. Sci. 2011, 53, 1825–1837. [Google Scholar] [CrossRef]
- Beni, A.; Ott, N.; Ura-Binczyk, E.; Rasinski, M.; Bauer, B.; Gille, P.; Ulrich, A.; Schmutz, P. Passivation and localised corrosion susceptibility of new Al–Cr–Fe complex metallic alloys in acidic NaCl electrolytes. Electrochim. Acta 2011, 56, 10524–10532. [Google Scholar] [CrossRef]
- McAllister, A.J. The Al-Co system. Bull. Alloy Phase Diagr. 1989, 10, 646–650. [Google Scholar] [CrossRef]
- Warmuzek, M. Metallographic Techniques for Aluminum and Its Alloys. In ASM Handbook, Vol. 9: Metallography and Microstructures; Vander Voort, G.F., Ed.; ASM Int.: Ohio, OH, USA, 2004; pp. 711–751. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical polarization I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–61. [Google Scholar] [CrossRef]
- Lekatou, A.; Zois, D.; Karantzalis, A.E.; Grimanelis, D. Electrochemical behaviour of cermet coatings with a bond coat on Al7075: Pseudopassivity, localized corrosion and galvanic effect considerations in a saline environment. Corros. Sci. 2010, 52, 2616–2635. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Silverman, D.C. Practical Corrosion Prediction Using Electrochemical Techniques. In Uhlig’s Corrosion Handbook, 3rd ed.; Revie, R.W., Ed.; Wiley & Sons: New York, NY, USA, 2011; pp. 1129–1166. [Google Scholar]
- Lekatou, A.; Sioulas, D.; Karantzalis, A.E.; Grimanelis, D. A comparative study on the microstructure and surface property evaluation of coatings produced from nanostructured and conventional WC-Co powders HVOF-sprayed on Al 7075. Surf. Coat. Tech. 2015, 276, 539–556. [Google Scholar] [CrossRef]
- Barclay, R.S.; Kerr, H.W.; Niessen, P. Off-eutectic composite solidification and properties in Al-Ni and Al-Co alloys. J. Mater. Sci. 1971, 6, 1168–1173. [Google Scholar] [CrossRef]
- Jackson, K.A. Crystal growth kinetics. Mater. Sci. Eng. 1984, 65, 7–13. [Google Scholar] [CrossRef]
- Jackson, K.A. The interface kinetics of crystal growth processes. Interface Sci. 2002, 10, 159–169. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.M. Growth morphologies and mechanism of TiC in the laser surface alloyed coating on the substrate of TiAl intermetallics. J. Alloy Compd. 2003, 351, 304–308. [Google Scholar]
- Karantzalis, A.E.; Lekatou, A.; Georgatis, E.; Arni, Z.; Dracopoulos, V. Solidification observations of vacuum arc melting processed FeAl-TiC composites: TiC precipitation mechanisms. Mater. Character. 2011, 62, 1196–1204. [Google Scholar] [CrossRef]
- Sater, J.M.; Sanders, T.H.; Garrett, R.K. Characterization of Rapidly Solidified Materials. In Rapidly Solidified Powder Aluminum Alloys; Fine, M.E., Starke, E.A., Eds.; ASTM STP 890, ASTM: Philadelphia, PA, USA, 1986; pp. 83–117. [Google Scholar]
- Sater, J.M.; Sanders, T.H. A Quantitative Microscopy Investigation of Melt Spun AI-10 wt.% Co. In Rapidly Solidified Materials; Lee, P.W., Carbonara, R.S., Eds.; ASM: Ohio, OH, USA, 1986; pp. 357–366. [Google Scholar]
- Bilušić, A.; Smiljanić, I.; Bihar, Ž.; Stanić, D.; Smontara, A. Heat Conduction in Complex Metallic Alloys. Croat. Chem. Acta 2010, 83, 21–25. [Google Scholar]
- Shen, J.; Liu, Y.; Han, Y.; Zhang, P.; Gao, H. Formation of bulk intermetallic compound Ag3Sn in slowly-cooled lead-free Sn-4.0 wt pct Ag solders. J. Mater. Sci. Technol. 2005, 21, 827–830. [Google Scholar]
- Wille, G.; Bourrat, X.; Maubec, N.; Guegan, R.; Lahfid, A. Raman-in-SEM Studies of Inorganic Materials. In Spectroscopic Properties of Inorganic and Organometallic Compounds: Techniques, Materials and Applications; Yarwood, J., Douthwaite, R., Duckett, S.B., Eds.; The Royal Society of Chemistry: London, UK, 2014; Volume 45, pp. 79–116. [Google Scholar]
- Osório, W.R.; Spinelli, J.E.; Ferreira, I.L.; Garcia, A. The roles of macrosegregation and of dendritic array spacings on the electrochemical behavior of an Al-4.5 wt.% Cu alloy. Electrochim. Acta 2007, 52, 3265–3273. [Google Scholar] [CrossRef]
- Robie, R.A.; Bruce, R.; Hemingway, S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures; U.S. Geological Survey Bulletin 2131; U.S. Department of the Interior: Washington DC, WA, USA, 1995. [Google Scholar]
- Hemingway, B.S.; Robie, R.A.; Kittrick, J.A. Revised values for the Gibbs free energy of formation of [Al(OH)4 aq-], diaspore, boehmite and bayerite at 298.15 K and 1 bar, the thermodynamic properties of kaolinite to 800 K and 1 bar, and the heats of solution of several gibbsite samples. Geochim. Cosmochim. Acta 1978, 42, 1533–1543. [Google Scholar] [CrossRef]
- Hem, J.D.; Roberson, C.E.; Lind, C.J. Thermodynamic stability of CoOOH and its coprecipitation with manganese. Geochim. Cosmochim. Acta 1985, 49, 801–810. [Google Scholar] [CrossRef]
- Wang, X.; Nie, M.; Wang, C.T.; Wang, S.C.; Gao, N. Microhardness and corrosion properties of hypoeutectic Al–7Si alloy processed by high-pressure torsion. Mater. Des. 2015, 83, 193–202. [Google Scholar]
- Santos, S.L.D.; Antunes, R.A.; Santos, S.F. Influence of injection temperature and pressure on the microstructure, mechanical and corrosion properties of a AlSiCu alloy processed by HPDC. Mater. Des. 2015, 88, 1071–1081. [Google Scholar]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASM Int.: Materials Park, Ohio, OH, USA, 2000; p. 328. [Google Scholar]
- Lekatou, A.; Karantzalis, A.E.; Evangelou, A.; Gousia, V.; Kaptay, G.; Gácsi, Z.; Baumli, P.; Simon, A. Aluminium reinforced by WC and TiC nanoparticles (ex-situ) and aluminide particles (in-situ): Microstructure, wear and corrosion behaviour. Mater. Des. 2015, 65, 1121–1135. [Google Scholar] [CrossRef]
- Ghali, E. Magnesium and Magnesium Alloys. In Uhlig’s Corrosion Handbook, 3rd ed.; Revie, R.W., Ed.; Wiley & Sons: New York, NY, USA, 2011; pp. 809–836. [Google Scholar]
- Harwood, J.J. The influence of stress on corrosion (part 1 of two parts). Corrosion 1950, 6, 249–259. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Mechanism of pit nucleation by electrical breakdown of the passive film. Corros. Sci. 2002, 44, 1143–1149. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Su, Y.; Mou, X.; Xiao, Y.; Liu, J. Investigation on the corrosion behavior of aluminum alloys 3A21 and 7A09 in chloride aqueous solution. Mater. Des. 2013, 50, 15–21. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Tabata, T.; Okada, O.; Sabatino, L.M.F.; Bellussi, G. A study on selective reduction of NOx by propane on Co-Beta. Catal. Lett. 1997, 44, 265–270. [Google Scholar] [CrossRef]
- Hunter, M.S.; Fowle, P. Naturally and thermally formed oxide films on aluminum. J. Electrochem. Soc. 1956, 103, 482–485. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Kapica, R.; Lojewska, J. Thin cobalt oxide films for catalysis deposited by plasma-enhanced metAl-organic chemical vapor deposition. Thin Solid Films 2007, 515, 6590–6595. [Google Scholar] [CrossRef]
- Vuurman, M.A.; Stufkens, D.J.; Oskam, A.; Deo, G.L.; Wachs, I.E. Combined Raman and IR study of MOX–V2O5/Al2O3 (MOx = MoO3, WO3, NiO, CoO) catalysts under dehydrated conditions. J. Chem. Soc. Faraday Trans. 1996, 92, 3259–3265. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. Comparison of Raman spectra in characterizing gibbsite, bayerite, diaspore and boehmite. J. Raman Spectrosc. 2001, 32, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hyldtoft, J.; Jacobsen, C.J. H; Nielsen, O.F. NIR FT Raman spectroscopic studies of γ-Al2O3 and Μο/γAl2O3 catalysts. Spectrochim. Acta 1995, 51A, 2161–2169. [Google Scholar] [CrossRef]
- Dyer, C.; Hendra, P.J.; Forsling, W.; Ranheimer, M. Surface hydration of aqueous γ-Al2O3 studied by Fourier transform Raman and infrared spectroscopy-I Initial results. Spectrochim. Acta 1993, 49A, 691–705. [Google Scholar] [CrossRef]
- Mariotto, G.; Cazzanelli, E.; Carturan, G.; Di Maggio, R.; Scardi, P. Raman and X-ray diffraction study of boehmite gels. J. Solid State Chem. 1990, 86, 263–274. [Google Scholar] [CrossRef]
- Kiss, A.B.; Keresztury, G.; Farkas, L. Raman and i.r. spectra and structure of boehmite (γ-AlOOH). Evidence for the recently discarded D172h space group. Spectrochim. Acta 1980, A36, 653–658. [Google Scholar] [CrossRef]
- Gwag, J.S.; Sohn, Y.K. Interfacial natures and controlling morphology of Co oxide nanocrystal structures by adding spectator Ni ions. Bull. Korean Chem. Soc. 2012, 33, 505–510. [Google Scholar] [CrossRef]
- Tang, C.W.; Wang, C.B.; Chien, S.H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Gallant, D.; Pézolet, M.; Simard, S. Inhibition of cobalt active dissolution by benzotriazole in slightly alkaline bicarbonate aqueous media. J. Phys. Chem. 2006, B110, 6871–6880. [Google Scholar] [CrossRef] [PubMed]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.N. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, 199–201. [Google Scholar] [CrossRef]
- Rao, V.S. A review of the electrochemical corrosion behaviour of iron aluminides. Electrochim. Acta 2004, 49, 4533–4542. [Google Scholar] [CrossRef]
- Palm, M.; Krieg, R. Neutral salt spray tests on Fe–Al and Fe–Al–X. Corros. Sci. 2012, 64, 74–81. [Google Scholar] [CrossRef]
- Foley, R.T. Localized corrosion of aluminum alloys—A review. Corrosion 1986, 42, 277–288. [Google Scholar] [CrossRef]











| Alloy (Al-wt.% Co) | Primary Al9Co2 (wt.%) | Eutectic Microconstituent | |
|---|---|---|---|
| αAl (wt.%) | Al9Co2 (wt.%) | ||
| Al-7Co | 19.4 | 78.5 | 2.1 |
| Al-10Co | 28.8 | 69.4 | 1.8 |
| Al-15Co | 44.5 | 54.1 | 1.4 |
| Al-20Co | 60.2 | 38.8 | 1.0 |
| Alloy Designation | Co in the Alloy (wt.%) | Al9Co2 in the Alloy (vol.%) | Co in Al9Co2 (at.%) | Max Co in αAl (wt.%) | 2-D Porosity (%) | Hardness (HB10) | Microhardness of αAl (HV1 gf/10 s) |
|---|---|---|---|---|---|---|---|
| Al1050-H14 (wrought) | – | – | – | – | – | 35 ± 1 | – |
| Al1050-cast 1 | – | – | – | – | – | 32 ± 0 | 33 ± 2 3 |
| Alpowder-VAR 2 | – | – | – | – | – | 39 ± 2 | 4 |
| Al-7 Co | 7.0 ± 0.5 | 36 ± 3 | 16.25 ± 1.19 | 0.59 | 0.33 ± 0.07 | 52 ± 4 | 57 ± 3 |
| Al-10 Co | 10.0 ± 0.5 | 41 ± 1 | 17.35 ± 0.55 | 1.89 | 0.40 ± 0.03 | 55 ± 6 | 71 ± 11 |
| Al-15 Co | 15.5 ± 1.5 | 50 ± 1 | 17.10 ± 0.86 | 1.82 | 0.57 ± 0.06 | 65 ± 6 | 76 ± 11 |
| Al-20 Co | 21.0 ± 2.0 | 63 ± 1 | 17.14 ± 1.35 | 5.51 | 0.59 ± 0.04 | 95 ± 10 | 94 ± 17 5 |
| Alloy | Ecorr (mV vs. Ag/AgCl) | Ea/c tr (mV vs. Ag/AgCl) | Eb (mV vs. Ag/AgCl) | Ecp (mV vs. Ag/AgCl) | Ea/c tr − Ecorr (mV) | Ecp − Ecorr (mV) | Eb − Ecp (mV) | Eb − Ecorr (mV) |
|---|---|---|---|---|---|---|---|---|
| Alpowder-VAR | −649 ± 11 | −711 ± 18 | −578 ± 13 | – | −62 ± 7 | – | – | 71 ± 2 |
| Al1050-VAR | −661 ± 19 | −701 ± 3 | −585 ± 14 | – | −40 ± 16 | – | – | 76 ± 5 |
| Al1050 cast | −682 ± 36 | −726 ± 12 | −628 ± 42 | – | −44 ± 24 | – | – | 54 ± 6 |
| Al1050-H14 | −657 ± 4 | −754 ± 19 | −634 ± 15 | – | −97 ± 15 | – | – | 23 ± 11 |
| Al-7 Co | −826 ± 36 | −655 ± 17 | −647 ± 12 | −772 ± 12 | 171 ± 19 | 54 ± 24 | 98 ± 0 | 179 ± 24 |
| Al-10 Co | −829 ± 23 | −641 ± 25 | −637 ± 25 | −763 ± 21 | 188 ± 2 | 66 ± 2 | 126 ± 4 | 192 ± 2 |
| Al-15 Co | −805 ± 23 | −643 ± 15 | −640 ± 15 | −755 ± 23 | 162 ± 8 | 50 ± 0 | 115 ± 8 | 165 ± 8 |
| Al-20 Co | −822 ± 22 | −627 ± 18 | −630 ± 8 | −763 ± 15 | 195 ± 4 | 59 ± 7 | 133 ± 7 | 192 ± 14 |
| Alloy (wt.% Co) | icorr (mA/cm2) | βc (mV/decade) | αc (mV) | rc2 | ΔE (mV vs. Ag/AgCl) | Δi (mA/cm2) | ip (mA/cm2) |
|---|---|---|---|---|---|---|---|
| Al-7 Co | 0.03 ± 0.01 | −140 ± 4 | −1022 ± 13 | 0.992 ± 0.003 | (−1021) − (−884) | 0.09 − 0.97 | 0.04 ± 0.01 |
| Al-10 Co | 0.06 ± 0.02 | −153 ± 11 | −1012 ± 11 | 0.987 ± 0.003 | (−1045) − (−892) | 0.14 − 1.64 | 0.08 ± 0.02 |
| Al-15 Co | 0.07 ± 0.02 | −163 ± 9 | −995 ± 11 | 0.986 ± 0.003 | (−1032) − (−869) | 0.15 − 1.5 | 0.09 ± 0.03 |
| Al-20 Co | 0.09 ± 0.02 | −175 ± 5 | −004 ± 25 | 0.984 ± 0.001 | (−1065) − (−895) | 0.15 − 1.54 | 0.12 ± 0.03 |
| Compound | ΔGof, 298 (kJ/mol) |
|---|---|
| α-Al2O3 | −1582 |
| γ-Al2O3 | −1563 |
| α-Al(OH)3 (bayerite) | −1153 |
| γ-Al(OH)3 (gibbsite) | −1155 |
| α-AlOOH (diaspore) | −923 |
| γ-AlOOH (boehmite) | −918 |
| Co3O4 | −775 |
| Co(OH)2 | −450 |
| CoOOH | −386 |
| CoO | −214 |
| Compound | Raman Wavenumbers (cm−1) | |||
|---|---|---|---|---|
| Al-O | γ(OH) | δ(OH) | Ref. | |
| Bayerite α-Al(OH)3 | 322–325, 387, 430–432 | 525–527, 532–533, 545–548, 767–771, 817–818, 898–899 | – | [69,70,71] |
| Gibbsite γ-Al(OH)3 | 320–321, 369–372, 377–379, 398–400, 410–413 | 539–541, 569–571, 816, 844–845, 893–895 | 924–925 | [69] |
| Diaspore α-AlOOH | 331–333, 392–396, 448–452 | 583–587, 654–658, 703–708, 786–790, 809, 836 | 911–913 | [69] |
| Boehmite γ-AlOOH | 348–349, 363–365, 499–500 | 636, 669-674, 731-735 | – | [69,70,72,73] |
| – | Eg | F2g | A1g | – |
| CO3O4 | 485–488 | 519–524, 617–622 | 685–689 | [39,67,74,75,76,77] |
| CoO | 463–470 | 508–510 | 672–674 | [39,75] |
| CoOOH | 476–480 | 602–606 | 804–806 | [75] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekatou, A.; Sfikas, A.K.; Petsa, C.; Karantzalis, A.E. Al-Co Alloys Prepared by Vacuum Arc Melting: Correlating Microstructure Evolution and Aqueous Corrosion Behavior with Co Content. Metals 2016, 6, 46. https://doi.org/10.3390/met6030046
Lekatou A, Sfikas AK, Petsa C, Karantzalis AE. Al-Co Alloys Prepared by Vacuum Arc Melting: Correlating Microstructure Evolution and Aqueous Corrosion Behavior with Co Content. Metals. 2016; 6(3):46. https://doi.org/10.3390/met6030046
Chicago/Turabian StyleLekatou, Angeliki, Athanasios K. Sfikas, Christina Petsa, and Alexandros E. Karantzalis. 2016. "Al-Co Alloys Prepared by Vacuum Arc Melting: Correlating Microstructure Evolution and Aqueous Corrosion Behavior with Co Content" Metals 6, no. 3: 46. https://doi.org/10.3390/met6030046
APA StyleLekatou, A., Sfikas, A. K., Petsa, C., & Karantzalis, A. E. (2016). Al-Co Alloys Prepared by Vacuum Arc Melting: Correlating Microstructure Evolution and Aqueous Corrosion Behavior with Co Content. Metals, 6(3), 46. https://doi.org/10.3390/met6030046