1. Introduction
Quality control in the production of canned fish is focused on the processes oriented to produce and preserve a uniform and acceptable product after storage in order to meet and comply with high standards, traceability and food safety conditions [
1].
In a supermarket where fish are offered in attractive and ready-to-cook units, color is one of the major attributes that affects the consumer perception of quality [
2]. Visual appearance is often the only criterion on which the consumer has to base his or her selection of fish purchase.
However, the quality of materials does not always follow such rigorous standards concerning environmental sustainability, and their life cycle rather becomes the responsibility of suppliers.
Selecting adequate, eco-efficient food containers with a good recycling performance is a key factor that plays a major role in environmental preservation, especially considering the large number of containers produced worldwide [
3]. Remarkable achievements for recycled PET (polyethylene terephthalate) can be found in several fields of application, such as medical vascular prostheses, agricultural textile films, hydrophobic membranes, traffic signs and paper bins, among others [
4]. The material employed in this study has been designed for use in salmon containers and consisted of a metal-polymer composite made up of a multilayer no more than 200 microns in total thickness.
The observations made in earlier studies have determined that a small percentage of salmon muscle adheres to the interior surface of container walls. Furthermore, these analyses have evaluated the mechanism of adhesion between the salmon muscle and the protective polymer, the influence of the degree of salmon freshness and the effect of the polymer surface failures on adhesion [
5]. From the research line above, the present work intends to determine the effects on the base steel-chromium layer interface and the changes in the multilayer container system; these being knowledge-intensive differentiating aspects, validated through a set of material characterization techniques.
PET coatings provide an improved protection barrier effect between the food and chromium layers deposited on the base steel of the container. The layers and metal substrate combine together to give abrasion and degradation resistance against aggressive electrolytes and structural rigidity to the can. The PET polymer is located in the internal wall of the container and contributes to preserving the organoleptic characteristics of food in time, preventing physicochemical interactions with the environment.
However, multilayer composites have complex behaviors, showing failures involving delamination, contamination and chemical degradation [
6]. Our hypothesis states that the adhesion of biopolymers or amino acids of food cans affects the polymer coating and the metal substrates in time and is an additional problem that can also decrease the performance of composites with regard to their functionality as a container.
Furthermore, an important aspect in salmon packaging is the production of energy in the postmortem muscle, where the oxygen supply to the muscle tissue is interrupted, and then, the energy production is restricted. Postmortem glycolysis results in the buildup of lactic acid in fish exposed to the stress of transport and/or slaughtering with the concomitant decrease in muscular pH in time [
7].
The manufacturing processes to ensure sufficient shelf-life of canned foods include heat-treating by steam, steam-air mixtures, water or spraying water. The amount of heating needed in the coldest spot, used as a reference point to prevent food spoilage and the presence of viable microorganisms, is regularly in the range 4–12 min at 121 °C for some typical canned food products [
8,
9,
10]. However, quality controls have detected the occurrence of food adhesion to the polymer-coated can walls arising from the application of these heat treatments; such adhesion has been linked to chemical bonding factors at the polymer interface level. It has been reported that proteins are the major cause of this adhesion [
11]. Proteins are large biological molecules consisting of chains of covalently-bound amino acids. The bonds formed between the amino acid residues can be of any of these four types: covalent, electrostatic, hydrogen and hydrophobic [
12].
A series of physicochemical processes occur inside the container during storage, inducing changes that affect the freshness of canned fish, and therefore, both the canning production processes and technologies play an important role in prolonging the useful life of end-user applications made up of composite materials [
8,
13,
14]. The chemical changes affecting the industrial molecules are strongly related to the crystallinity of polymers and occur especially with the sterilization temperatures employed in the canning processes; thus, extreme environmental conditions may degrade PET coatings [
15,
16] and expose the underlying metal substrates.
This study is intended to determine the salmon-PET polymer interactions, to evaluate the effects on multilayers, to characterize the degradation or failures resulting from the adhesion of salmon muscle to the polymer coating surface and to assess the microstructural changes undergone, correlating all results through low-dimension techniques to estimate the impact on the container’s functionality that may limit its useful life and its recycling capacity.
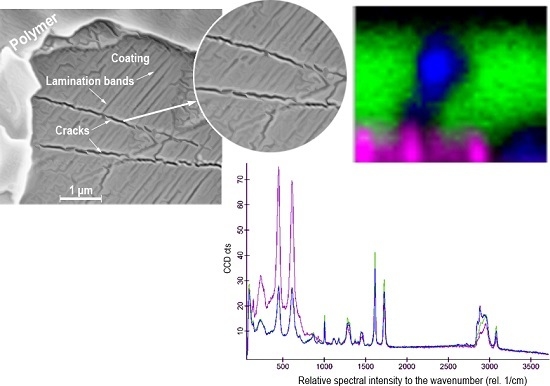
The damage in localized points of the polymer surface by the physicochemical adhesion of salmon muscle to the can wall is increased by the manufacturing defects of the container, reaching the multilayers and producing partial morphological and structural degradation, which are key aspects in quality control systems.
Failure analysis work on food containers is worthy both to consumers and manufacturers to preserve the environment and for recycling purposes, respectively.
2. Materials and Methods
The material employed in this study is an eco-efficient, environmentally-friendly, chromium(VI)-free (non-carcinogenic) metal-polymer. This thin, multilayered electrolytic chromium-coated steel (ECCS) laminate protected by a polyethylene terephthalate (PET) coating is employed in the salmon canning industry. The food container employed in this study was manufactured in one of the main canning factories in Chile, a country whose aquaculture industry has grown to become the second largest producer of salmon commercialized worldwide. The chemical composition of steel employed in the manufacturing of food cans for this work consisted of 0.074% C, 0.260% Mn, 0.021% P, 0.016% S, 0.012% Si, 0.032% Al, 45 ppm N and Fe (remaining percentage).
The steel laminate was protected by a coating generated by electrolytic deposition and consists of 0.01 μm-thick chromium Cr0 and chromium oxide Cr2O3 with an average thickness of 0.01 μm and a total chromium content of 101.33 mg/m2. This electrolytic chromium-coated steel (ECCS) plate had a 30 µm-thick layer of polyethylene terephthalate (PET) consisting of polymerized units of dimethyl terephthalate and ethylene glycol, applied on the surface as a protective barrier against the canned food content. The biaxial-structure PET polymer coating was applied as a film bath on the ECCS sheet.
The metal-polymer salmon cans were manufactured employing 50 mL of a 2.5% NaCl solution, sterilized at 120 °C for 60 min, immersed in a warm water bath in the range of 50–80 °C prior to sterilization. The containers under study were stored for different time spans from 1 week to 14 months at 20 °C before opening. The experimental study evaluated salmon adhesion in those cans stored for 14 months; the average percentage of adhesion for the samples analyzed was 2.5%–3.5% of the total weight after complete emptying of the can in the laboratory (
Figure 1).
Later, the removal of salmon muscle adhered to the can walls was performed with 6 mol/L urea solution to form hydrogen bonds and unfold the proteins. The most representative cans, concerning the areas of salmon adhesion and stored for a period ranging between 12 and 14 months, were selected. The effects of urea and the changes on the multilayers were evaluated and characterized in the areas of the greatest concentration of failures or evident morphological changes on the polymer surface.
The metal-polymer substrate was characterized by X-ray diffraction (XRD) with a D2 Phaser diffractometer (Bruker Corporation, Bruker, Germany). The equipment employs Ni-filtered Cu radiation (30 kV and 10 mA), a 1-mm anti-scatter slit, a 2.5° Soller slit and a LYNXEYE™ detector. The alignment is regularly checked against the NIST Standard Reference Materials (SRM) 1976 alumina plate standard. Patterns were collected in the 5–70° range, counting 5 s per steps of 0.01°.
The surface characterization of salmon adhesion on the PET polymer was carried out by SEM using a LEO 400 series scanning electron microscope (Zeiss, Jena, Germany) and confocal Raman imaging with a spectral resolution at 532 nm to determine the distribution of the different components on the sample.
All Raman images and spectra were recorded using an alpha 300 RA confocal microscope (Zeiss, Jena, Germany) equipped with a CCD camera, an Ultrahigh Throughput Spectrometer (UHTS), frequency-doubled Nd (YAG laser used for 532 nm excitation) and a Zeiss 100× air objective (Numerical Aperture = 0.9). The camera and spectrometer parameters are listed below; CCD Camera DU970_UVB; spectrometer UHTS300; the measurements were acquired using the WITec Control FOUR software (WITec, Jena, Germany) and analyzed with the WITec Project FOUR (WITec, Jena, Germany) and the WITec Project FOUR Plus software (WITec, Jena, Germany), respectively. Points in the directions X–Y and X–Z evidencing color changes in the samples were analyzed.
The multilayer composite samples were analyzed by micro-Raman spectroscopy (B&W Tek Inc., Newark, NY, USA). The equipment employed to provide information on the samples consisted of two back-illuminated fiber-coupled spectrometers featuring 633-nm and 785-nm wavelengths. The experimental procedure of this technique considered mainly the data gathered with the 785-nm spectrometer. This device consisted of a multi-mode fiber laser BWTEK BRM-OEM-785 (785 nm), a Raman head BWTEK BAC100-785E and an objective lens Zeiss Epiplan 50×/0.50 infinite/0 44 28 50 M20.32 mm (focal length). The maximum output of the laser through this lens was approximately 165 mW; the laser spot diameter measured on the samples was 48 microns. The spectrometer was a BWTEK Prime T BTC661E-785CUST, covering a spectral range of 0–3000 cm−1, with a resolution of 5 cm−1 and a CCD Hamamatsu S10141-1107S operating at −10 °C. Over 20 surface spots showing evidence of failure were analyzed to determine the chemical and orientation changes of the molecular chains and/or atomic rearrangements, in order to facilitate the understanding of the mechanisms associated with localized polymer failures caused by the adhesion of salmon muscle to the PET coating in time.
4. Discussion
The localized PET polymer degradation characterized by SEM and caused by the salmon muscle adhesion in time shows evidence of physicochemical interactions occurring in the system. As a result of the above, the chromium layers are exposed and partially degraded, especially in the direction of the steel laminate, altering the composite’s performance, since the multilayers cease to play their protective role. Regarding the chromium layers, specifically in those areas exhibiting damage, they do not perform their passive function of protecting the base steel, which may in turn lead to corrosion processes.
The cause of this degradation originated in the organic material or biopolymer, which is mainly composed of proteins and lipids [
19]. Lipid oxidation processes occur as a series of reactions in the free-radical chains and lead to complex chemical changes that negatively affect the quality of food [
20], such as the accumulation of lactic acid from postmortem glycolysis and decreased pH [
21].
Other studies [
22,
23] have found that 1% lactic acid on chromium-passivated tin plates and protected by organic compounds can be attacked by corrosion currents due to the presence of pores and interfacial defects that allow degradation and loss of functionality at pH 2–3. The postmortem salmon produces lactic acid and may be one of the sources of organic acid that may act on the container-food system.
The application of hydrophobic products, such as organosilane precursors employed in other applications, may be an option to neutralize and minimize the effect of the adhesion characterized in this work [
24].
Thus, our future studies should be focused on determining the concentrations of lactic acid in canned salmon in time and on measuring the effects on the ECCS-PET under controlled potentiostatic conditions. This will allow elucidating the timing of physicochemical interactions for the localized salmon adhesion to the polymer surface of the can wall and the effects on the properties of the multilayer composite.
On the other hand, the spectroscopy analyses showed a correlation with the SEM observations regarding the presence of not only surface changes, but damage to the layers involving microstructural and functional changes of the multilayers, either by atomic rearrangements or phase changes.
The spectroscopy results showed clear differences between the spectra acquired from samples treated with urea solution and those from the control Sample 1 exhibiting commonly-occurring natural adhesion. Some other bands of urea appear as a shoulder or small contributions to the functional groups of PET. It is interesting to note that the main vibrational bands of the PET coating remained practically unaltered under this urea solution treatment employed to denature and detach the salmon muscle.
It is particularly important to emphasize that the spectra of Samples 1 and 2, taken from areas with hardly any PET degradation, are different from those of areas showing evidence of damage.
However, the spectral region 2700–3000 cm
−1 (
Figure 8), corresponding to ν(C–H) vibrations, presents important differences in intensity.
Concerning
Figure 6,
Figure 8 and
Figure 9, the vibrational spectroscopy analyses show differences in the band at 1200 cm
−1; corresponding to changes in polymer crystallinity, atomic rearrangements and stretching of chains mainly observable in the bands at 960 cm
−1 and 1300 cm
−1, as well as adhesion of proteins and peptides to the protective polymer coating in the range of 2900–3200 cm
−1.
Detailed information on the origin and processing practices of salmon muscle for canning and on proteomic studies of these samples will allow us to identify the compounds formed by the denaturation of the proteins adhered and thermally affected by the sterilization process during canning.