Experimental Investigation of the Ti-Nb-Sn Isothermal Section at 1173 K
Abstract
:1. Introduction
2. Experimental Details
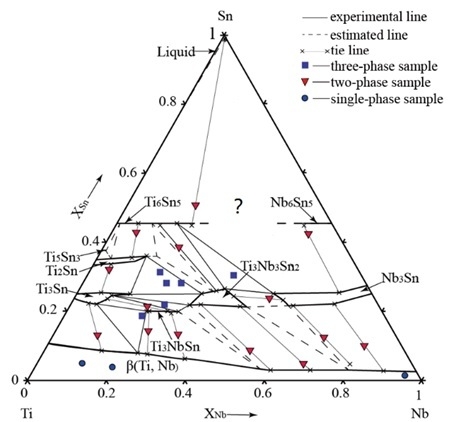
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Niinomi, M.; Kobayashi, T. Fracture Characteristics Analysis Related to the Microstructures in Titanium Alloys. Mater. Sci. Eng. A 1996, 213, 231–236. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Syu, J.Y.; Ho, W.F. The Structure and Mechanical Properties of As-cast Ti-25Nb-xSn Alloys for Biomedical Applications. Mater. Sci. Eng. A 2013, 568, 1–7. [Google Scholar] [CrossRef]
- Guo, Y.; Georgarakis, K.; Yokoyama, Y.; Yavari, A.R. On the Mechanical Properties of TiNb Based Alloys. J. Alloys Comp. 2013, 571, 25–30. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Yang, R. Effect of Zr and Sn on Young’s Modulus and Superelasticity of Ti-Nb-based Alloys. Mater. Sci. Eng. A. 2006, 441, 112–118. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, J.J.; Guo, Y.; Georgarakis, K.; Yavari, A.R.; Evangelakis, G.A.; Lekka, C.E. The Role of Sn Doping in the β-type Ti-25 at. % Nb Alloys: Experiment and ab Initio Calculations. J. Alloys Comp. 2014, 615, 676–679. [Google Scholar] [CrossRef]
- Murray, J.L. Phase Diagram of Binary Titanium Alloys; ASM International: Materials Park, OH, USA, 1987; pp. 294–299. [Google Scholar]
- Kuper, C.; Peng, W.; Pisch, A.; Goesmann, F.; Schmid-Fetzer, R. Phase Formation and Reaction Kinetics in the System Ti-Sn. Z. Metallkunde 1998, 89, 855–862. [Google Scholar]
- Liu, C.; Klotz, U.E.; Uggowitzer, P.J.; Löffler, J.F. Thermodynamic Assessment of the Sn-Ti System. Monatsh. Chem. 2005, 136, 1921–1930. [Google Scholar] [CrossRef]
- Yin, F.; Tedenac, J.C.; Gascoin, F. Thermodynamic Modelling of the Ti-Sn System and Calculation of the Co-Ti-Sn System. Calphad 2007, 31, 370–379. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Leinenbach, C.; Klotz, U.E.; Uggowitzer, P.J.; Löffler, J.F. Experimental Investigation and Thermodynamic Assessment of the Cu-Sn-Ti Ternary System. Calphad 2011, 35, 82–94. [Google Scholar] [CrossRef]
- Colinet, C.; Tedenac, J.C.; Fries, S.G. Constitutional and Thermal Defects in D019-SnTi3. Intermetallics 2008, 16, 923–932. [Google Scholar] [CrossRef]
- Colinet, C.; Tedenac, J.C. Constitutional and Thermal Defects in B82-SnTi2. Intermetallics 2009, 17, 291–304. [Google Scholar] [CrossRef]
- Toffolon, C.; Servant, C.; Sundman, B. Thermodynamic Assessment of the Nb-Sn System. J. Phase Equilib. 1998, 19, 479–485. [Google Scholar] [CrossRef]
- Toffolon, C.; Servant, C.; Gachon, J.C.; Sundman, B. Reassessment of the Nb-Sn System. J. Phase Equilib. 2002, 23, 134–139. [Google Scholar] [CrossRef]
- Li, M.; Du, Z.M.; Guo, C.P.; Li, C.R. Thermodynamic Optimization of the Cu-Sn and Cu-Nb-Sn Systems. J. Alloys Comp. 2009, 477, 104–117. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Liu, H.S.; Jin, Z.P. Thermodynamic Assessment of the Nb-Ti System. Calphad 2001, 25, 305–317. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, L.B.; Zhang, X.D.; Bai, W.M.; Chen, C.P. Isothermal Section of the Ti-Nb-Sn Ternary System at 700 °C. J. Phase Equilib. 2014, 35, 223–231. [Google Scholar] [CrossRef]
- Watanabe, K.; Awaji, S.; Katagiri, K.; Noto, K.; Goto, K.; Sugimoto, M.; Saito, T.; Kohno, O. Highly Strengthened Multifilamentary (Nb, Ti)3Sn Wires Stabilized with CuNb Composite. IEEE Trans. Magn. 1994, 30, 1871–1874. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamada, Y.; Sakuraba, J.; Hata, F.; Chong, C.K.; Hasebe, T.; Ishihara, M. (Nb, Ti)3Sn Superconducting Magnet Operated at 11 K in Vacuum Using High-Tc (Bi, Pb)2Sr2Ca2Cu3O10 Current Leads. Jpn. J. Appl. Phys. 1993, 32, 488–490. [Google Scholar] [CrossRef]
- Kikuchi, A.; Taniguchi, H.; Yoshida, Y.; Tomonaga, M.; Takeuchi, T. New Ti-Sn Intermetallic Compound and (Nb, Ti)3Sn Conductor. IEEE Trans. Appl. Supercond. 2009, 19, 2556–2559. [Google Scholar] [CrossRef]
- Holleck, H.; Nowotny, H.; Benesovsky, F. Über das Mischungsverhalten von Nb3Sn mit Ti3Sn, Mo3Al und verwandten Phasen. Monatsh. Chem. 1963, 94, 359–365. [Google Scholar] [CrossRef]
- OʼBrien, J.W.; Dunlap, R.A.; Dahn, J.R. A Mössbauer Effect and X-ray Diffraction Investigation of Ti-Sn Intermetallic Compounds: II. Nanostructured Phases Prepared by Ball Milling with Al2O3 and TiN. J. Alloys Comp. 2003, 353, 60–64. [Google Scholar] [CrossRef]
- Okamoto, H. Sn-Ti(Tin-Titanium). J. Phase Equilib. Diffus. 2010, 31, 202–203. [Google Scholar] [CrossRef]





| Alloy | Compositions (at. %) | Phase | Compositions (at. %) | ||
|---|---|---|---|---|---|
| Ti | Nb | Sn | |||
| 1 | Ti83.6Nb11.3Sn5.1 | β(Ti, Nb) | 83.7 | 11.3 | 5.0 |
| 2 | Ti76.6Nb19.6Sn3.8 | β(Ti, Nb) | 76.5 | 19.6 | 3.9 |
| 3 | Ti3.4Nb95.1Sn1.5 | β(Ti, Nb) | 3.4 | 95.1 | 1.5 |
| 4 | Ti75.7Nb11.2Sn13.1 | Ti3Sn | 73.3 | 4.3 | 22.4 |
| β(Ti, Nb) | 76.9 | 14.5 | 8.6 | ||
| 5 | Ti62.2Nb23.5Sn14.3 | Ti3NbSn | 59.0 | 20.8 | 20.2 |
| β(Ti, Nb) | 65.8 | 26.5 | 7.7 | ||
| 6 | Ti55.2Nb31.6Sn13.2 | Ti3NbSn | 53.8 | 26.8 | 19.4 |
| β(Ti, Nb) | 57.0 | 36.8 | 6.2 | ||
| 7 | Ti39.2Nb52.1Sn8.7 | Ti3Nb3Sn2 | 45.0 | 32.9 | 22.1 |
| β(Ti, Nb) | 36.7 | 60.3 | 3.0 | ||
| 8 | Ti27.5Nb67.8Sn4.7 | Ti3Nb3Sn2 | 35.3 | 43.2 | 21.5 |
| β(Ti, Nb) | 26.7 | 70.3 | 3.0 | ||
| 9 | Ti18.4Nb69.1Sn12.5 | Nb3Sn | 21.7 | 56.6 | 21.7 |
| β(Ti, Nb) | 15.8 | 79.5 | 4.7 | ||
| 10 | Ti9.3Nb80.6Sn10.1 | Nb3Sn | 10.9 | 66.8 | 22.23 |
| β(Ti, Nb) | 8.2 | 89.1 | 2.7 | ||
| 11 | Ti58.9Nb19.7Sn21.4 | Ti3Sn | 67.8 | 8.5 | 23.7 |
| Ti3NbSn | 54.3 | 25.6 | 20.1 | ||
| 12 | Ti26.7Nb49.6Sn23.7 | Ti3Nb3Sn2 | 35.3 | 40.2 | 24.5 |
| Nb3Sn | 35.4 | 22.5 | 42.1 | ||
| 13 | Ti23.3Nb53.2Sn23.5 | Ti2Sn | 62.0 | 4.3 | 33.7 |
| Ti3Sn | 68.6 | 5.9 | 25.5 | ||
| 14 | Ti50.9Nb6.2Sn42.9 | Ti6Sn5 | 49.0 | 5.4 | 45.6 |
| Ti2Sn | 55.7 | 8.7 | 35.6 | ||
| 15 | Ti42.2Nb19.2Sn38.6 | Ti6Sn5 | 43.9 | 11.1 | 45.0 |
| Ti3Nb3Sn2 | 39.0 | 35.1 | 25.9 | ||
| 16 | Ti7.5Nb50.1Sn42.4 | Nb6Sn5 | 7.3 | 47.7 | 45.0 |
| Nb3Sn | 8.1 | 66.4 | 25.5 | ||
| 17 | Ti32.0Nb17.3Sn50.7 | Liquid Sn | 0.3 | 0.6 | 99.1 |
| Ti6Sn5 | 35.8 | 19.3 | 45.0 | ||
| 18 | Ti61.5Nb19.7Sn18.8 | Ti3Sn | 71.5 | 6.4 | 22.1 |
| Ti3NbSn | 59.7 | 20.2 | 20.2 | ||
| β(Ti, Nb) | 68.0 | 24.0 | 8.0 | ||
| 19 | Ti54.2Nb23.9Sn21.9 | Ti3NbSn | 51.6 | 28.5 | 19.9 |
| Ti3Nb3Sn2 | 47.8 | 30.34 | 21.9 | ||
| Ti3Sn | 66.2 | 9.1 | 24.7 | ||
| 20 | Ti50.6Nb17.9Sn31.5 | Ti2Sn | 51.7 | 12.2 | 36.1 |
| Ti3Sn | 61.4 | 13.5 | 25.1 | ||
| Ti3Nb3Sn2 | 43.2 | 31.6 | 25.2 | ||
| 21 | Ti50.7Nb21.1Sn28.2 | Ti2Sn | 51.7 | 12.2 | 36.1 |
| Ti3Sn | 61.4 | 13.5 | 25.1 | ||
| Ti3Nb3Sn2 | 43.2 | 31.6 | 25.2 | ||
| 22 | Ti46.8Nb24.8Sn28.4 | Ti2Sn | 51.7 | 12.2 | 36.1 |
| Ti3Sn | 61.4 | 13.5 | 25.1 | ||
| Ti3Nb3Sn2 | 43.2 | 31.6 | 25.2 | ||
| 23 | Ti32.3Nb37.1Sn30.6 | Ti6Sn5 | 39.3 | 15.2 | 45.5 |
| Ti3Nb3Sn2 | 36.6 | 36.8 | 26.6 | ||
| Nb3Sn | 24.9 | 49.9 | 25.2 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, L.; Zhang, L.; Tuo, B.; Bai, W.; Wu, D. Experimental Investigation of the Ti-Nb-Sn Isothermal Section at 1173 K. Metals 2016, 6, 60. https://doi.org/10.3390/met6030060
Wang J, Liu L, Zhang L, Tuo B, Bai W, Wu D. Experimental Investigation of the Ti-Nb-Sn Isothermal Section at 1173 K. Metals. 2016; 6(3):60. https://doi.org/10.3390/met6030060
Chicago/Turabian StyleWang, Jianli, Libin Liu, Ligang Zhang, Biyang Tuo, Weimin Bai, and Di Wu. 2016. "Experimental Investigation of the Ti-Nb-Sn Isothermal Section at 1173 K" Metals 6, no. 3: 60. https://doi.org/10.3390/met6030060
APA StyleWang, J., Liu, L., Zhang, L., Tuo, B., Bai, W., & Wu, D. (2016). Experimental Investigation of the Ti-Nb-Sn Isothermal Section at 1173 K. Metals, 6(3), 60. https://doi.org/10.3390/met6030060