Effects of Basicity and MgO in Slag on the Behaviors of Smelting Vanadium Titanomagnetite in the Direct Reduction-Electric Furnace Process
Abstract
:1. Introduction
2. Materials and Experimental
2.1. Materials
2.2. Thermodynamics Analysis Methods
2.3. Experimental Procedure
2.4. Definition of Parameters
2.4.1. Basicity
2.4.2. Recovery Rates
2.5. Analysis of Samples
3. Experimental Fundamental Thermodynamics
4. Results and Discussion
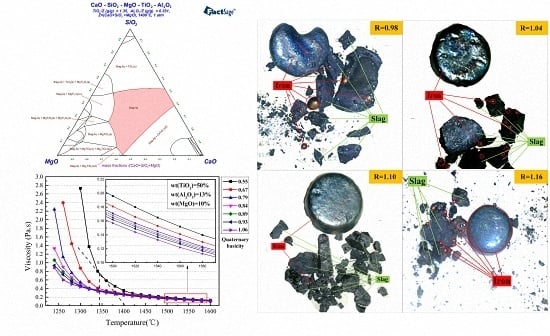
4.1. Effects of Basicity
4.2. Effects of MgO
5. Conclusions
- (1).
- The related thermodynamic analysis indicated that the suitable basicity range was 0.9–1.2. The addition of MgO decreased titanium slag viscosity but increased its melting temperature and free-running temperature.
- (2).
- A higher level of basicity benefits the reduction of iron oxides and vanadium oxides, and titanium content dropped in molten iron with the increased basicity. The optimum basicity was about 1.1. As the content of MgO increased, the recovery rate of Fe increased slightly, but the recovery rate of V considerably increased. The grades of Ti in molten iron were at a low level without significant change when MgO content was below 11%, but increased as MgO content increased to 12.75%. MgO content of 11.38% and basicity of 1.1 were the optimum conditions in this study.
- (3).
- The vanadium-bearing iron and titanium slag were produced at 1550 °C and 20 min, with 5% reducing agent. The recovery rates of Fe and V were 99.59% and 95.52%, respectively. The titanium slag contained 50.34% TiO2. The vanadium-bearing iron can be applied in the converter steelmaking process and titanium slag can be utilized by the acid leaching method.
Author Contributions
Conflicts of Interest
References
- Zheng, F.Q.; Chen, F.; Guo, Y.F.; Jiang, T.; Travyanov, A.Y.; Qiu, G.Z. Kinetics of Hydrochloric Acid Leaching of Titanium from Titanium-Bearing Electric Furnace Slag. JOM 2016, 68, 1476–1484. [Google Scholar] [CrossRef]
- Taylor, P.R.; Shuey, S.A.; Vidal, E.E.; Gomez, J.C. Extractive metallurgy of vanadium-containing titaniferous magnetite ores: A review. Miner. Metall. Proc. 2006, 23, 80–86. [Google Scholar]
- Du, H.G. Principle of Blast Furnaces Melting Vanadium-Titanium Magnetite; Science Press: Beijing, China, 1996; pp. 6–8. [Google Scholar]
- Tan, Q.Y.; Chen, B.; Zhang, Y.S.; Long, Y.B.; Yang, Y.H. Characteristics and Current Situation of Comprehensive Utilization of Vanadium Titanomagnetite Resources in Panxi Region (In Chinese). Multipurp. Util. Miner. Resour. 2011, 6, 6–10. [Google Scholar]
- Chen, S.Y.; Chu, M.S. A new process for the recovery of iron, vanadium, and titanium from vanadium titanomagnetite. J. South. Afr. Inst. Min. Metall. 2014, 114, 481–488. [Google Scholar]
- Samanta, S.; Mukherjee, S.; Dey, R. Upgrading Metals via Direct Reduction from Poly-metallic Titaniferous Magnetite Ore. JOM 2015, 67, 467–476. [Google Scholar] [CrossRef]
- Fu, W.G.; Wen, Y.C.; Xie, H.E. Development of intensified technologies of vanadium-bearing titanomagnetite smelting. J. Iron Steel Res. Int. 2011, 18, 7–18. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, D.S.; Chu, J.L.; Wang, W.J.; Li, Y.L.; Qi, T. Recovery of titanium and vanadium from titanium–vanadium slag obtained by direct reduction of titanomagnetite concentrates. Rare Met. 2015. [Google Scholar] [CrossRef]
- Guo, Y.F. Study on Strenthening of Solid-State Reduction and Comprehensive Utilization of Vanadiferous Titanomagnetite (In Chinese). Ph.D. Thesis, Central South University, Changsha, China, July, 2007. [Google Scholar]
- Jones, J.A.; Bowman, B.; Lefrank, P.A. Electric Furnace Steelmaking. Available online: http://jpkc.gsut.edu.cn/upload/20120523/20120523181249992.pdf (accessed on 6 May 2016).
- Tang, J.; Chu, M.; Xue, X. Optimized use of MgO flux in the agglomeration of high-chromium vanadium-titanium magnetite. Int. J. Miner. Metall. Mater. 2015, 22, 371–380. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, M.; Wang, H.; Zhao, W.; Xue, X. Effect of MgO content in sinter on the softening-melting behavior of mixed burden made from chromium-bearing vanadium-titanium magnetite. Int. J. Miner. Metall. Mater. 2016, 23, 25–32. [Google Scholar] [CrossRef]
- Chen, M.; Raghunath, S.; Zhao, B. Viscosity Measurements of SiO2-“FeO”-MgO System in Equilibrium with Metallic Fe. Metall. Mater. Trans. B 2014, 45, 58–65. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Hong, C.; Ma, X.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9wt%Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Li, P.; Ning, X. Effects of MgO/Al2O3 Ratio and Basicity on the Viscosities of CaO-MgO-SiO2-Al2O3 Slags: Experiments and Modeling. Metall. Mater. Trans. B 2016, 47, 446–457. [Google Scholar]
- Fan, J.J.; Cai, M.X.; Zhang, H. Study on effects of Al2O3 content and MgO content on melting temperature at BF slag (In Chinese). Shanxi Metall. 2007, 30, 22–23. [Google Scholar]
- Shankar, A.; Rnerup, M.R.G.; Lahiri, A.K.; Seetharaman, S. Experimental Investigation of the Viscosities in CaO-SiO2-MgO-Al2O3 and CaO-SiO2-MgO-Al2O3-TiO2 Slags. Metall. Mater. Trans. B 2007, 38, 911–915. [Google Scholar] [CrossRef]
- He, H.Y.; Wang, Q.X.; Zeng, X.N. Effect of MgO content on BF slag viscosity (In Chinese). J. Iron Steel Res. 2006, 18, 11–13. [Google Scholar]
- Li, J.; Zhang, Z.; Liu, L.; Wang, W.; Wang, X. Influence of Basicity and TiO2 Content on the Precipitation Behavior of the Ti-bearing Blast Furnace Slags. ISIJ Int. 2013, 53, 1696–1703. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; Pelton, A.D.; et al. FactSage thermochemical software and databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Huang, X.H. Principles of Iron and Steel Metallurgy; Metallurgy Industry Press: Beijing, China, 2013; pp. 60–440. [Google Scholar]
- Zhen, Y.; Zhang, G.; Chou, K. Viscosity of CaO-MgO-Al2O3-SiO2-TiO2 Melts Containing TiC Particles. Metall. Mater. Trans. B 2015, 46, 155–161. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Lou, T.P.; Sui, Z.T.; Jang, J.S. Effect of perovskite phase precipitation on viscosity of Ti-bearing blast furnace slag under the dynamic oxidation condition. J. Non-Cryst. Solids 2006, 352, 123–129. [Google Scholar] [CrossRef]
- Yang, S.L. Non-Blast Furnace Smelting Technology for Vanadium Titanomagnetite Ore; Metallurgy Industry Press: Beijing, China, 2012. [Google Scholar]















| TFe | MFe | CaO | SiO2 | MgO | Al2O3 | V2O5 | TiO2 | C | S |
|---|---|---|---|---|---|---|---|---|---|
| 71.38 | 64.56 | 1.01 | 3.71 | 2.58 | 3.83 | 0.82 | 15.53 | 0.036 | 0.027 |
| No. | Binary Basicity | Quaternary Basicity | MgO/% | Smelting Temperature/°C | Smelting Time/min | Reducing Agent/% |
|---|---|---|---|---|---|---|
| 1 | 0.8 | 0.98 | 11.38 | 1550 | 20 | 2 |
| 2 | 0.9 | 0.99 | 11.38 | 1550 | 20 | 2 |
| 3 | 1.0 | 1.04 | 11.38 | 1550 | 20 | 2 |
| 4 | 1.1 | 1.10 | 11.38 | 1550 | 20 | 2 |
| 5 | 1.2 | 1.16 | 11.38 | 1550 | 20 | 2 |
| 6 | 1.1 | 0.88 | 7.97 | 1550 | 20 | 2 |
| 7 | 1.1 | 0.96 | 9.79 | 1550 | 20 | 2 |
| 8 | 1.1 | 1.13 | 12.75 | 1550 | 20 | 2 |
| 9 | 1.1 | 1.10 | 11.38 | 1550 | 20 | 5 |
| Element Grade in Iron (wt. %) | Recovery Rate/% | ||||
|---|---|---|---|---|---|
| TFe | V | Si | Ti | V | Fe |
| 98.09 | 0.683 | 0.199 | 0.296 | 95.52 | 99.59 |
| TiO2 | V2O5 | TFe | Al2O3 | MgO | CaO | SiO2 |
|---|---|---|---|---|---|---|
| 50.34 | 0.162 | 1.051 | 13.05 | 11.38 | 11.79 | 11.68 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Wang, S.; Guo, Y.; Chen, F.; Zheng, F. Effects of Basicity and MgO in Slag on the Behaviors of Smelting Vanadium Titanomagnetite in the Direct Reduction-Electric Furnace Process. Metals 2016, 6, 107. https://doi.org/10.3390/met6050107
Jiang T, Wang S, Guo Y, Chen F, Zheng F. Effects of Basicity and MgO in Slag on the Behaviors of Smelting Vanadium Titanomagnetite in the Direct Reduction-Electric Furnace Process. Metals. 2016; 6(5):107. https://doi.org/10.3390/met6050107
Chicago/Turabian StyleJiang, Tao, Shuai Wang, Yufeng Guo, Feng Chen, and Fuqiang Zheng. 2016. "Effects of Basicity and MgO in Slag on the Behaviors of Smelting Vanadium Titanomagnetite in the Direct Reduction-Electric Furnace Process" Metals 6, no. 5: 107. https://doi.org/10.3390/met6050107
APA StyleJiang, T., Wang, S., Guo, Y., Chen, F., & Zheng, F. (2016). Effects of Basicity and MgO in Slag on the Behaviors of Smelting Vanadium Titanomagnetite in the Direct Reduction-Electric Furnace Process. Metals, 6(5), 107. https://doi.org/10.3390/met6050107