Author Contributions
Mohamed Tawfik and Arvind Kumar supplied the samples and gathered the performance data on their pots; Bénédicte Allard analyzed the paste samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allard, B.; Paulus, R.; Billat, G. A new ramming paste with improved potlining working conditions. Light Met. 2011, 1091–1096. [Google Scholar] [CrossRef]
- Doornaert, B.; Pichard, A. Hydrocarbures Polycycliques Aromatiques (HAPs); Final report 18 December 2003; INERIS: France, 2003. [Google Scholar]
- Allard, B.; Dreyfus, J.M.; Lenclud, M. Evolution of thermal, electrical and mechanical properties of graphitized cathode blocks for aluminium electrolysis cells with temperature. Light Met. 2000, 515–521. [Google Scholar]
Figure 1.
Green density curves versus number of strokes for the five pastes.
Figure 2.
Relation between maximum density given by the Fischer Sand Rammer (FSR) and the apparent green density given by the hand rammer.
Figure 3.
Relation between volume expansion and baked density.
Figure 4.
Relation between flexural strength and crushing strength.
Figure 5.
Equipment to follow-up PAH and VOC emissions during paste baking.
Figure 6.
Example of a kinetic curve of ethylbenzene and benzene emissions in paste C.
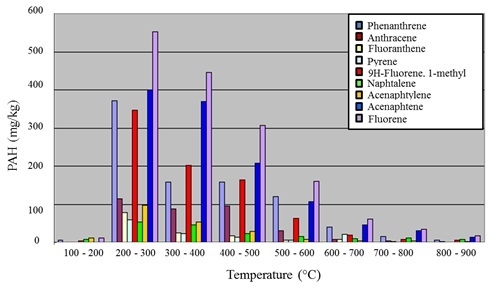
Figure 7.
Emission of gaseous PAH versus temperature for paste C.
Figure 8.
Cell resistance weekly average values ((in micro Ohm)) for pots with different pastes.
Figure 9.
Line amperage average weekly values (in kA) for pots with different pastes.
Figure 10.
Noise voltage average weekly values (in Volts) for pots with different pastes.
Figure 11.
Cathode resistance average weekly values (in micro Ohm) for pots with different pastes.
Figure 12.
Pot age distribution for DUBAL pots with NeO2/CleO2 pastes, October 2015.
Figure 13.
Cleaned D18 pot cavity with NeO2 paste at 680 days.
Figure 14.
Cleaned DX pot cavity with CleO2 paste at 369 days.
Figure 15.
Preheat temperature for DX pots with different pastes.
Table 1.
Working temperature range and expiry dates of the five pastes studied.
| Sample ID | A | B | C | D | E |
|---|
| T range (°C) | 20 to 40 | 10 to 50 | 17 to 42 | 20 to 40 | 10 to 50 |
| Expiry date | End of September 2015 | End of July 2015 | End of Jane 2016 | End of July 2015 | End of March 2016 |
Table 2.
Polycyclic aromatic hydrocarbons (PAH), benzene, toluene, ethylbenzene, xylene (BTEX), and volatile organic compounds (VOC) composition of the pastes in mg/kg.
| Component (mg/kg) | A | B | C | D | E |
|---|
| Fluoranthene | 1529 | <10 | 1817 | 1255 | <10 |
| Benzo(b + j)fluoranthene | 54.1 | <10 | 37.2 | 1745 | <10 |
| Benzo(k)fluoranthene | 16.7 | <10 | 11.2 | 392 | <10 |
| Benzo(a)pyrene | 59.1 | <10 | 38.4 | 1373 | <10 |
| Indeno(1,2,3)pyrene | 69.2 | <10 | 53.8 | 459 | <10 |
| Benzo(g,h,i)perylene | 143 | <10 | 88.5 | 1294 | <10 |
| Naphtalene | 663 | <10 | 492 | 14,137 | <10 |
| Acenaphtene | 13,394 | <10 | 16,481 | 13,196 | <10 |
| Fluorene | 7798 | <10 | 12,327 | 2863 | <10 |
| Phenantrene | 4683 | <10 | 5587 | 882 | <10 |
| Pyrene | 808 | <10 | 1087 | 1088 | <10 |
| Benzo(a)anthracene | 51.6 | <10 | 59.7 | 892 | <10 |
| Chrysene | 45.7 | <10 | 54.9 | 1088 | <10 |
| Acenaphtylene | <10 | <10 | 12.9 | 31.7 | <10 |
| Dibenzo(a,h)anthracene | 21.5 | <10 | 18.3 | 190 | <10 |
| Anthracene | 541 | <10 | 554 | 155 | <10 |
| Benzo(b)naphto(2,1-d)thiophene | <10 | <10 | 10.1 | 122 | <10 |
| PAH total | 29,877 | - | 38,730 | 41,163 | - |
| Benzene | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Toluene | <0.05 | <0.05 | 0.23 | <0.05 | <0.05 |
| Ethylbenzene | 0.15 | <0.05 | 0.55 | 0.21 | <0.05 |
| Xylene | 0.19 | <0.10 | 0.54 | 0.61 | <0.10 |
| BTEX total | 0.34 | - | 0.78 | 0.82 | - |
| dichloromethane | 0.38 | 0.31 | 0.37 | 0.4 | 0.32 |
| trimethyl-1,2,4 benzene | 0.07 | <0.05 | 0.1 | 0.68 | <0.05 |
| trimethyl-1,3,5 benzene | 0.1 | <0.05 | 0.18 | 1.1 | <0.05 |
| n-butyl benzene | 0.14 | <0.05 | 0.2 | 0.26 | <0.05 |
| cumene | <0.05 | <0.05 | 0.1 | 0.08 | <0.05 |
| n-propyl benzene | <0.05 | <0.05 | 0.06 | <0.05 | <0.05 |
| styrene | <0.25 | <0.25 | <0.25 | 0.28 | <0.25 |
| cymene | <0.05 | <0.05 | <0.05 | 0.1 | <0.05 |
Table 3.
Relative potency factor [2].
| PAH | Relative Potency Factor (RPF) |
|---|
| Naphtalene (NA) | - |
| Fluorene (FL) | 0.001 |
| Phenantrene (PH) | 0.001 |
| Anthracene (ANT) | 0.01 |
| Fluoranthene (FA) | 0.001 |
| Pyrene (PY) | 0.001 |
| Benzo(a)anthracene (BaA) | 0.1 |
| Chrysene (CH) | 0.01 |
| Benzo(b)fluoranthene (BbFA) | 0.1 |
| Benzo(k)fluoranthene (BkFA) | 0.1 |
| Benzo(a)pyrene (BaP) | 1 |
| Benzo(g,h,i)perylene (BghiP) | 0.01 |
| Dibenzo(a,h)anthracene (DiahA) | 1 |
| Indeno(1,2,3)pyrene (IP) | 0.1 |
Table 4.
Equivalent toxicity factor according to [2] for pastes A, C, and D.
| Type of Paste | A | C | D | Tepid | Cold | Tepid Eco-Friendly (EF) |
|---|
| Equivalent toxicity factor (ETF) (ppm) | 121.9 | 100.7 | 1943 | 2500 | 2300 | 300 |
Table 5.
Rammability index N2 and maximum density (ρmax).
| Type of Paste | A | B | C | D | E |
|---|
| N2 | 63 | 97 | 123 | 104 | 138 |
| ρmax (g/cm3) | 1.591 | 1.654 | 1.632 | 1.742 | 1.646 |
Table 6.
Average hydrostatic apparent green density (HAD) for each paste (g/cm3).
| Type of Paste | A | B | C | D | E |
|---|
| HAD (g/cm3) | 1.608 | 1.640 | 1.610 | 1.674 | 1.645 |
Table 7.
Comparison of paste properties after baking.
| Paste | Baked Density | Weight Loss (%) | Volume Expansion (%) | Thermal Conductivity (W/m·K) | Real Density | Crushing Strength (MPa) | Flexural Strength (MPa) | Young’s Modulus (GPa) | Open Porosity (%) | Total Porosity (%) | Electrical Resistivity (µΩ·m) | Ash Content (%) | Oxidation (%) |
|---|
| A | 1.450 | 9.7 | 0.2 | 5.0 | 1.857 | 12.7 | 2.1 | 3.6 | 19.1 | 20.8 | 92.0 | 6.6 | 6.6 |
| A’ | 1.443 | 9.6 | 0.7 | - | - | - | - | - | - | - | - | - | - |
| B | 1.431 | 12.2 | 0.6 | 5.0 | 1.825 | 17.4 | 3.3 | 7.3 | 18.8 | 20.6 | 70.4 | 3.3 | 4.5 |
| B’ | 1.428 | 12.2 | 0.8 | - | - | - | - | - | - | - | - | - | - |
| C | 1.468 | 8.8 | −0.1 | 7.5 | 1.862 | 15.6 | 2.9 | 4.2 | 18.3 | 20.6 | 70.0 | 2.9 | 4.5 |
| C’ | 1.464 | 9.0 | 0.1 | - | - | - | - | - | - | - | - | - | - |
| D | 1.539 | 9.3 | −1.2 | 12.1 | 1.976 | 19.9 | 4.1 | 4.9 | 19.0 | 21.6 | 47.7 | 4.6 | 9.9 |
| D’ | 1.516 | 9.8 | −0.5 | - | - | - | - | - | - | - | - | - | - |
| E | 1.403 | 11.9 | 3.3 | 7.5 | 1.938 | 18.2 | 4.3 | 7.3 | 22.6 | 25.8 | 48.6 | 2.3 | 5.2 |
| E’ | 1.381 | 12.6 | 4.1 | - | - | - | - | - | - | - | - | - | - |
Table 8.
Thermal conductivity and electrical resistivity at 1000 °C.
| Type of Paste | A | B | C | D | E |
|---|
| Thermal conductivity at 1000 °C (W/m·K) | 11.9 | 13.1 | 13.9 | 16.2 | 13.9 |
| Electrical resistivity at 1000 °C (µΩ·m) | 67 | 52 | 48 | 36 | 37 |
Table 9.
Detection limits for VOC. µGC: micro gas chromatography; TCD: thermal conductivity detector; MS: mass spectrometer.
| Molecules | Methods of Measurement | Detection Limit per Molecule (ppm) |
|---|
| O2, N2 | μGC/TCD | 100 |
| Methane, CO | μGC/TCD | 40 |
| H2, CO2, light VOC C2 to C3 | μGC/TCD | 5 |
| Light VOC C3 to C9 | μGC/TCD/MS | 1 |
Table 10.
PAH and ETF comparison. LD: Limit of detection.
| Component (mg/kg) | A | B | C | D | E |
|---|
| FA | 507 | - | 284 | 553 | - |
| BbjFA | - | - | - | - | - |
| BkFA | 200 | - | 39 | 665 | - |
| BaP | 158 | - | 50 | 302 | - |
| IP | 37 | - | - | - | - |
| BghiP | 136 | - | 11 | - | - |
| NA | 680 | 50 | 174 | 1548 | 59 |
| AC | 4138 | 11 | 2104 | 1744 | 20 |
| FL | 2114 | 15 | 2546 | 338 | 33 |
| PH | 1439 | 12 | 1379 | 351 | 26 |
| PY | 452 | - | 233 | 501 | - |
| BaA | 3 | - | - | 97 | - |
| CH | 3 | - | - | 20 | - |
| ACY | 297 | - | 306 | 404 | - |
| DiahA | 84 | - | 20 | 17 | - |
| ANT | 326 | - | 403 | 199 | 7 |
| BbNT | - | - | - | - | - |
| PAH sum | 10573 | 88 | 7549 | 6738 | 146 |
| ETF | 251 | 0 | 82 | 323 | 0 |
| CO2 | <LD | 12391 | 224 | 1315 | 13182 |
| CH4 | 1383 | 1287 | 673 | 805 | 1306 |
| CO | 679 | 5823 | 818 | 1609 | 5868 |
| H2 | 181 | 813 | - | 2106 | 742 |
| Permanent gas | 2244 | 20314 | 1715 | 5834 | 21098 |
| Total VOC | 84 | 5166 | 133 | 101 | 6009 |
| BTEX | 50 | 169 | 133 | 71 | 130 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).