Microstructure Evolution and High-Temperature Compressibility of Modified Two-Step Strain-Induced Melt Activation-Processed Al-Mg-Si Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructural Evolution
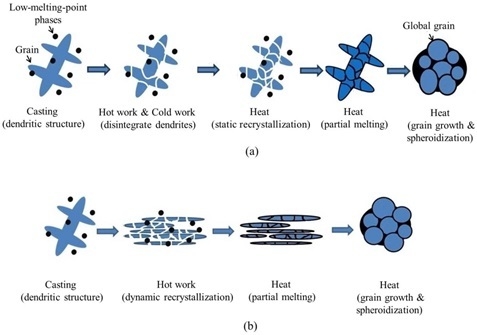
3.2. Formation Mechanism for the Two-Step Strain-Induced Melt Activation (TS-SIMA) Process
3.3. High-Temperature Compression of TS-SIMA-Processed Alloy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy; ASM International: Materials Park, OH, USA, 1984; Volume 1, p. 50. [Google Scholar]
- Zhen, L.; Fei, W.D.; Kang, S.B.; Kim, H.W. Precipitation behavior of Al-Mg-Si alloys with high silicon content. J. Mater. Sci. 1997, 32, 1895–1902. [Google Scholar] [CrossRef]
- Laughlm, D.E.; Miao, W.F. The effect of Cu and Mn content and processing on precipitation hardening behavior in Al-Mg-Si-Cu alloy 6022. Miner. Met. Mater. Soc. 1998, 63–78. [Google Scholar]
- Mondolfo, L.F. Aluminum Alloys Structure & Properties; Butterworths: London, UK, 1976; pp. 806–842. [Google Scholar]
- Fan, Z. Semisolid metal processing. Int. Mater. Rev. 2002, 47, 49–85. [Google Scholar] [CrossRef]
- Song, Y.B.; Park, K.T.; Hong, C.P. Recrystallization behavior of 7175 Al alloy during modified strain-induced melt-activated (SIMA) process. Mater. Trans. 2006, 47, 1250–1256. [Google Scholar] [CrossRef]
- Tzimas, E.; Zavaliangos, A. A comparative characterization of near-equiaxed microstructures as produced by spray casting, magnetohydrodynamic casting and the stress induced, melt activated process. Mater. Sci. Eng. A 2000, 289, 217–227. [Google Scholar] [CrossRef]
- Paes, M.; Zoqui, E.J. Semi-solid behavior of new Al-Si-Mg alloys for thixoforming. Mater. Sci. Eng. A 2005, 406, 63–73. [Google Scholar] [CrossRef]
- Parshizfard, E.; Shabestari, S.G. An investigation on the microstructural evolution and mechanical properties of A380 aluminum alloy during SIMA process. J. Alloy. Compd. 2011, 509, 9654–9658. [Google Scholar] [CrossRef]
- Akhlaghi1, F.; Farhood, A.H.S. Characterization of globular microstructure in NMS processed aluminum A356 alloy: The role of casting size. Adv. Mater. Res. 2011, 264–265, 1868–1877. [Google Scholar] [CrossRef]
- Tzimas, E.; Zavaliangos, A. Evolution of near-equiaxed microstructure in the semisolid state. Mater. Sci. Eng. A 2000, 289, 228–240. [Google Scholar] [CrossRef]
- Emamy, M.; Razaghian, A.; Karshenas, M. The effect of strain-induced melt activation process on the microstructure and mechanical properties of Ti-refined A6070 Al alloy. Mater. Des. 2013, 46, 824–836. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, S.; Lim, K.R.; Hong, S.H.; Kim, K.B.; Na, Y.S. Crystallization, high temperature defroemtaion behavior and solid-to-dolid formability of a Ti-based bulk metallic glass within supercooled liquid region. J. Alloy. Compd. 2016, 663, 270–278. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, Z.; Liu, J.; Ma, X. Preparation of a high strength Al-Cu-Mg alloy by mechanical alloying and press-forming. Mater. Sci. Eng. A 2012, 550, 51–54. [Google Scholar] [CrossRef]
- Katayama, T.; Nakamachi, E.; Nakamura, Y.; Ohata, T.; Morishita, Y.; Murase, H. Development of process design system for press forming multi-objective optimization of intermediate die shape in transfer forming. J. Mater. Process. Technol. 2004, 155–156, 1564–1570. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, Z.; Hu, M.; Xu, H. Evolution of the semi-solid microstructure of ADC12 alloy in a modified SIMA process. Mater. Charact. 2011, 62, 925–930. [Google Scholar] [CrossRef]
- Yan, G.; Zhao, S.; Ma, S.; Shou, H. Microstructural evolution of A356.2 alloy prepared by the SIMA process. Mater. Charact. 2012, 69, 45–51. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. High-temperature deformation and forming behavior of two-step SIMA-processed 6066 ally. Mater. Sci. Eng. A 2016, 659, 143–157. [Google Scholar] [CrossRef]
- Bolouri, A.; Shahmiri, M.; Kang, C.G. Coarsening of equiaxed microstructure in the semisolid state of aluminum 7075 alloy through SIMA processing. J. Mater. Sci. 2012, 47, 3544–3553. [Google Scholar] [CrossRef]
- Hardy, S.C.; Voorhees, P.W. Ostwald ripening in a system with a high volume of coarsening phase. Metall. Trans. A 1988, 19, 2713–2721. [Google Scholar] [CrossRef]
- ASM International Alloy Phase Diagram and the Handbook Committees. Alloy Phase Diagram, ASM Handbook 3; ASM International: Materials Park, OH, USA, 1992; pp. 307–308. [Google Scholar]
- Zhang, L.; Liu, Y.B.; Cao, Z.Y.; Zhang, Y.F.; Zhang, Q.Q. Effects of isothermal process parameters on the microstructure of semisolid AZ91D alloy produced by SIMA. J. Mater. Process. Technol. 2009, 209, 792–797. [Google Scholar] [CrossRef]
- Qin, Q.D.; Zhao, Y.G.; Xiu, K.; Zhou, W.; Liang, Y.H. Microstructure evolution of in situ Mg2Si/Al-Si-Cu composite in semisolid remelting processing. Mater. Sci. Eng. A 2005, 407, 196–200. [Google Scholar] [CrossRef]
















| Element | Mg | Si | Cu | Mn | Fe | Cr | Al |
|---|---|---|---|---|---|---|---|
| Mass% | 1.02 | 1.29 | 0.98 | 1.02 | 0.19 | 0.18 | Balanced |
| Specimen | SB3/610 | SB3/620 | SB3/630 |
|---|---|---|---|
| K (μm3·min−1) | 10751 | 16806 | 50820 |
| R2 | 0.9852 | 0.9926 | 0.9818 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Hung, F.-Y.; Lui, T.-S.; Chen, L.-H. Microstructure Evolution and High-Temperature Compressibility of Modified Two-Step Strain-Induced Melt Activation-Processed Al-Mg-Si Aluminum Alloy. Metals 2016, 6, 113. https://doi.org/10.3390/met6050113
Lin C-W, Hung F-Y, Lui T-S, Chen L-H. Microstructure Evolution and High-Temperature Compressibility of Modified Two-Step Strain-Induced Melt Activation-Processed Al-Mg-Si Aluminum Alloy. Metals. 2016; 6(5):113. https://doi.org/10.3390/met6050113
Chicago/Turabian StyleLin, Chia-Wei, Fei-Yi Hung, Truan-Sheng Lui, and Li-Hui Chen. 2016. "Microstructure Evolution and High-Temperature Compressibility of Modified Two-Step Strain-Induced Melt Activation-Processed Al-Mg-Si Aluminum Alloy" Metals 6, no. 5: 113. https://doi.org/10.3390/met6050113
APA StyleLin, C. -W., Hung, F. -Y., Lui, T. -S., & Chen, L. -H. (2016). Microstructure Evolution and High-Temperature Compressibility of Modified Two-Step Strain-Induced Melt Activation-Processed Al-Mg-Si Aluminum Alloy. Metals, 6(5), 113. https://doi.org/10.3390/met6050113