Formation and Disruption of W-Phase in High-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Heat Treatment
2.2. Material Characterization
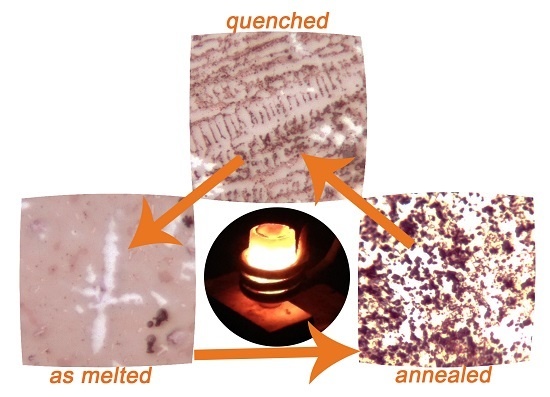
3. Results
4. Discussion
5. Conclusions
- The high concentration of scandium required by equimolarity brings forth the formation of intermetallic compounds, preventing the formation of a single phase. Among them, formation of Al2Cu3Sc compound has been detected for the first time in a multicomponent alloy.
- The synthesis of a single-phase Al, Cu and Sc containing HEA is not compatible with melting from powders, due to the formation of stable intermetallic compounds.
- The product of the W-phase degradation following heat-treatment is a combination of intermetallics in a soft hcp-Sc/Ti alloy matrix. The thermally annealed alloy has increased hardness (890 HV) and crack resistance.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SEM-EDX | Scanning electrode microscope equipped with energy dispersed X-ray analyzer |
| AFM | Atomic force microscopy |
| SKP | Scanning Kelvin probe |
| PXRD | Powder X-ray diffraction |
References
- Samaei, A.T.; Mirsayar, M.M.; Aliha, M.R.M. Microstructure and mechanical behaviour of modern high temerature alloys. Eng. Sol. Mech. 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Yakushchenko, I.V.; Beresnev, V.M. The structure and properties of high-entropy alloys and nitride coatings based on them. Russ. Chem. Rev. 2014, 83, 1027–1061. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Pradeep, K.G.; Wanderka, N.; Choi, P.; Banhart, J.; Murty, B.S.; Raabe, D. Atomic-scale compositona characterization of a nanocrystalline AlCrCuFeNiZn high-entropy alloy using atom probe tomography. Acta Mater. 2013, 61, 4696–4706. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Xiao, H.; Zhou, L.; Zhu, D.; Yang, S. Fabrication and properties of nanocrystalline Co0.5FeNiCrTi0.5 high entropy alloy by MA-SPS technique. Mater. Des. 2013, 44, 535–539. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Kong, J.; Zhang, J.; Li, T. Microstructure and mechanical properties of multi-component AlCrFeNiMox high-entropy alloys. J. Alloy. Compd. 2013, 537, 96–101. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Song, X.; Ye, F.; Lin, J.; Cheng, G. Microstructure and performance of TiAlBeSc alloys with low density. Rare Met. Mater. Eng. 2012, 5, 826–829. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cho, Y.H. Elucidating the microstructural and tribological characteristics of NiCrAlCoCu and NiCrAlCoMo multicomponent alloy cladlayers synthesized in situ. Surf. Coat. Technol. 2009, 203, 1694–1701. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.K.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distribution of a multi-principal-element alloy. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Riva, S.; Yusenko, K.Y.; Lavery, N.P.; Jarvis, D.J.; Brown, S.G.R. The scandium effect in multicomponent alloys. Int. Mater. Rev. 2016. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 2766. [Google Scholar]
- Anstis, G.R.; Chantikul, P.; Lawn, B.R.; Marshall, D.B. A critical evaluation of indentation techniques for measuring fracture toughness: I, Direct crack measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Kharakterova, M.L. Phase composition of Al-Cu-Sc alloys at temperatures of 450 and 500 °C. Russ. Metall. 1991, 4, 195–199. [Google Scholar]
- Gazizov, M.; Teleshov, V.; Zakharov, V.; Kaibyshev, R. Solidification behaviour and the effects of homogenisation on the structure of an Al-Cu-Mg-Ag-Sc alloy. J. Alloy. Compd. 2011, 509, 9497–9507. [Google Scholar] [CrossRef]
- Norman, A.F.; Prangnell, P.B.; McEwen, R.S. The solidification behaviour of dilute aluminium-scandium alloys. Acta Mater. 1998, 46, 5715–5732. [Google Scholar] [CrossRef]




| AlCrCuScTi | Average Composition (±0.5 at. %) from 2.5 mm × 1.5 mm Maps | Density (±0.02 g·cm−3) | Hardness | |||||
|---|---|---|---|---|---|---|---|---|
| Al | Cr | Cu | Sc | Ti | HV | MPa | ||
| Nominal | 20 | 20 | 20 | 20 | 20 | 4.71 (estimated) | - | - |
| As melted | 21.7 | 20.0 | 21.6 | 17.4 | 19.3 | 4.84 | 636 ± 27 | 6237 ± 267 |
| Annealed | 16.7 | 19.1 | 19.5 | 23.3 | 23.3 | 4.83 | 890 ± 20 | 8728 ± 196 |
| Quenched | 18.1 | 22.3 | 20.2 | 20.5 | 20.8 | - | 797 ± 10 | 7816 ± 98 |
| Phase | As-Melted Alloy | Annealed Alloy |
|---|---|---|
| fcc AuCu3-type | Yes | Traces |
| fcc AuCu-type | Yes | No |
| bcc (I) Cr | Yes | No |
| bcc (II) | Yes | Traces |
| hcp (I) | Yes | Yes |
| hcp (II) | No | Yes |
| Al4Cu4Sc | Yes | Traces |
| Al2Cu3Sc | Yes | Traces |
| Al3Sc | Traces | Traces |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, S.; Fung, C.M.; Searle, J.R.; Clark, R.N.; Lavery, N.P.; Brown, S.G.R.; Yusenko, K.V. Formation and Disruption of W-Phase in High-Entropy Alloys. Metals 2016, 6, 106. https://doi.org/10.3390/met6050106
Riva S, Fung CM, Searle JR, Clark RN, Lavery NP, Brown SGR, Yusenko KV. Formation and Disruption of W-Phase in High-Entropy Alloys. Metals. 2016; 6(5):106. https://doi.org/10.3390/met6050106
Chicago/Turabian StyleRiva, Sephira, Chung M. Fung, Justin R. Searle, Ronald N. Clark, Nicholas P. Lavery, Stephen G. R. Brown, and Kirill V. Yusenko. 2016. "Formation and Disruption of W-Phase in High-Entropy Alloys" Metals 6, no. 5: 106. https://doi.org/10.3390/met6050106
APA StyleRiva, S., Fung, C. M., Searle, J. R., Clark, R. N., Lavery, N. P., Brown, S. G. R., & Yusenko, K. V. (2016). Formation and Disruption of W-Phase in High-Entropy Alloys. Metals, 6(5), 106. https://doi.org/10.3390/met6050106