Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing
Abstract
:1. Introduction
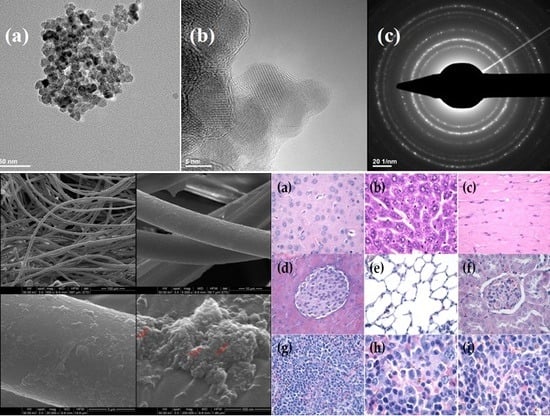
2. Results
3. Discussion
4. Materials and Method
4.1. Materials
4.2. Fabrication of Nanoparticles and Preparation of Modified Wound Dressing
4.3. Characterization Methods
4.3.1. XRD
4.3.2. TEM
4.3.3. SEM
4.3.4. IR
4.3.5. Cell Viability
4.3.6. In Vivo Biodistribution
4.3.7. Biofilm Assay
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolcott, R. Disrupting the biofilm matrix improves wound healing outcomes. J. Wound Care 2015, 24, 366–371. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, S.; Al-Kurdi, D.; Ologun, Y.; Ovington, L.G. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Clinton, A.; Carter, T. Chronic wound biofilms: Pathogenesis and potential therapies. Lab. Med. 2015, 46, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat. Anti-Infect. Drug Discov. 2010, 5, 10–17. [Google Scholar] [CrossRef]
- Francolini, I.; Taresco, V.; Crisante, F.; Martinelli, A.; D’llario, L.; Ilario, L.; Piozzi, A. Water soluble usnic acid-polyacrylamide complexes with enhanced antimicrobial activity against staphylococcus epidermidis. Int. J. Mol. Sci. 2013, 14, 7356–7369. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Vriesekoop, F.; Yuan, Q.; Liang, H. Effects of nisin on the antimicrobial activity of d-limonene and its nanoemulsion. Food Chem. 2014, 150, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Saviuc, C.M.; Drumea, V.; Olariu, L.; Chifiriuc, M.C.; Bezirtzoglou, E.; Lazar, V. Essential oils with microbicidal and antibiofilm activity. Curr. Pharm. Biotechnol. 2015, 16, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.; Denny, C.; Benso, B.; de Alencar, S.; Rosalen, P. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Holban, A.M.; Andronescu, E.; Mogosanu, G.D.; Vasile, B.S.; Chifiriuc, M.C.; Lazar, V.; Andrei, E.; Constantinescu, A.; Maniu, H. Anionic polymers and 10 nm Fe3O4@UA wound dressings support human foetal stem cells normal development and exhibit great antimicrobial properties. Int. J. Pharm. 2014, 463, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Laz, R.V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.M.; Andronescu, E.; Anghel, A.G.; Ficai, A.; Saviuc, C.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. Magnetite nanoparticles for functionalized textile dressing to prevent fungal biofilms development. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.C.; dos Santos, M.M.; Lima, N.G.; Cidral, T.A.; Melo, M.C.; Lima, K.C. Prevalence and factors associated with wound colonization by Staphylococcus spp. And Staphylococcus aureus in hospitalized patients in inland northeastern brazil: A cross-sectional study. BMC Infect. Dis. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Kaye, K.S. Staphylococcal surgical site infections. Infect. Dis. Clin. North Am. 2009, 23, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Costa, L.B.; Silva, J.L.; Maia, M.B.; Ximenes, E.C. Interventions for wound healing among diabetic patients infected with Staphylococcus aureus: A systematic review. Sao Paulo Med. J. 2011, 129, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules 2013, 18, 9334–9351. [Google Scholar] [CrossRef] [PubMed]
- Faoagali, J.; George, N.; Leditschke, J.F. Does tea tree oil have a place in the topical treatment of burns? Burns 1997, 23, 349–351. [Google Scholar] [CrossRef]
- Sevgi, M.; Toklu, A.; Vecchio, D.; Hamblin, M.R. Topical antimicrobials for burn infections—An update. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 161–197. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yang, S.P.; Liu, W.Q. Evaluation of the antibacterial activity of patchouli oil. Iran. J. Pharm. Res. 2013, 12, 307–316. [Google Scholar] [PubMed]
- Grumezescu, A.; Gestal, M.; Holban, A.; Grumezescu, V.; Vasile, B.; Mogoantă, L.; Iordache, F.; Bleotu, C.; Mogoșanu, G. Biocompatible Fe3O4 increases the efficacy of amoxicillin delivery against gram-positive and gram-negative bacteria. Molecules 2014, 19, 5013–5027. [Google Scholar] [CrossRef] [PubMed]
- Fufa, M.O.; Mihaiescu, D.E.; Mogoanta, L.; Balseanu, T.A.; Mogosanu, G.D.; Grumezescu, A.M.; Bolocan, A. In vivo biodistribution of cnts using a balb/c mouse experimental model. Rom. J. Morphol. Embryol. 2015, 56, 1481–1493. [Google Scholar] [PubMed]
- Istrate, C.M.; Holban, A.M.; Grumezescu, A.M.; Mogoanta, L.; Mogosanu, G.D.; Savopol, T.; Moisescu, M.; Iordache, M.; Vasile, B.S.; Kovacs, E. Iron oxide nanoparticles modulate the interaction of different antibiotics with cellular membranes. Rom. J. Morphol. Embryol. 2014, 55, 849–856. [Google Scholar] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rădulescu, M.; Andronescu, E.; Holban, A.M.; Vasile, B.S.; Iordache, F.; Mogoantă, L.; Mogoșanu, G.D.; Grumezescu, A.M.; Georgescu, M.; Chifiriuc, M.C. Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing. Metals 2016, 6, 103. https://doi.org/10.3390/met6050103
Rădulescu M, Andronescu E, Holban AM, Vasile BS, Iordache F, Mogoantă L, Mogoșanu GD, Grumezescu AM, Georgescu M, Chifiriuc MC. Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing. Metals. 2016; 6(5):103. https://doi.org/10.3390/met6050103
Chicago/Turabian StyleRădulescu, Marius, Ecaterina Andronescu, Alina Maria Holban, Bogdan Stefan Vasile, Florin Iordache, Laurențiu Mogoantă, George Dan Mogoșanu, Alexandru Mihai Grumezescu, Mihaela Georgescu, and Mariana Carmen Chifiriuc. 2016. "Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing" Metals 6, no. 5: 103. https://doi.org/10.3390/met6050103
APA StyleRădulescu, M., Andronescu, E., Holban, A. M., Vasile, B. S., Iordache, F., Mogoantă, L., Mogoșanu, G. D., Grumezescu, A. M., Georgescu, M., & Chifiriuc, M. C. (2016). Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing. Metals, 6(5), 103. https://doi.org/10.3390/met6050103