Microstructure, Hardness Evolution, and Thermal Stability Mechanism of Mechanical Alloyed Cu-Nb Alloy during Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
3. Results
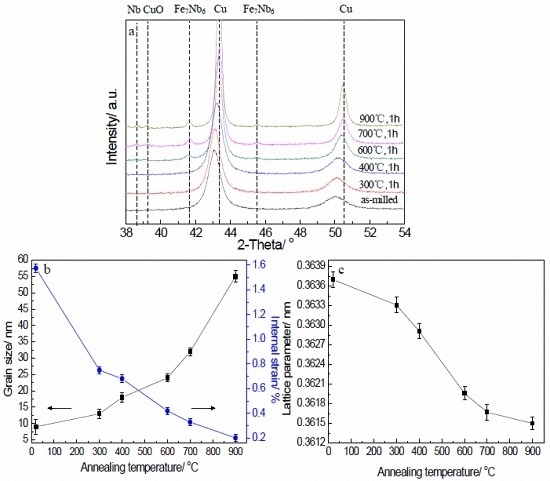
3.1. XRD Analysis
3.2. SEM Observations
3.3. TEM Observations
3.4. Microhardness Measurements
4. Discussion
4.1. Stability of Nb Nanoparticles
4.2. Stability of the Nanocrystalline Cu Matrix
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Z.M.; Fu, L.M.; Fu, B.; Shan, A.D. Effects of annealing on microstructure and mechanical properties of nano-grained titanium produced by combination of asymmetric and symmetric rolling. Mater. Sci. Eng. A 2012, 558, 309–318. [Google Scholar] [CrossRef]
- Herzer, G. Modern soft magnets: Amorphous and nanocrystalline materials. Acta Mater. 2013, 61, 718–734. [Google Scholar] [CrossRef]
- Azabou, M.; Khitouni, M.; Kolsi, A. Characterization of nanocrystalline Al-based alloy produced by mechanical milling followed by cold-pressing consolidation. Mater. Charact. 2009, 60, 499–505. [Google Scholar] [CrossRef]
- Lari Baghal, S.M.; Amadeh, A.; Sohi, M.H. Investigation of mechanical properties and operative deformation mechanism in nano-crystalline Ni-Co/SiC electrodeposits. Mater. Sci. Eng. A 2012, 542, 104–112. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Garroni, S.; Enzoa, S.; Delogu, F. Mesostructural refinement in the early stages of mechanical alloying. Scr. Mater. 2014, 83, 49–52. [Google Scholar] [CrossRef]
- Garroni, S.; Soru, S.; Enzo, S.; Delogu, F. Reduction of grain size in metals and metal mixtures processed by ball milling. Scr. Mater. 2014, 88, 9–12. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Chookajorn, T.J.; Murdoch, H.A.; Schuh, C.A. Design of Stable Nanocrystalline Alloys. Science 2012, 337, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Atwater, M.A.; Roy, D.; Darling, K.A.; Butler, B.G.; Scattergood, R.O.; Koch, C.C. The thermal stability of nanocrystalline copper cryogenically milled with tungsten. Mater. Sci. Eng. A 2012, 558, 226–233. [Google Scholar] [CrossRef]
- Akbarpour, M.R.; Kim, H.S. Microstructure, grain growth, and hardness during annealing of nanocrystalline Cu powders synthesized via high energy mechanical milling. Mater. Des. 2015, 83, 644–650. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhang, C.; Xia, Z.X.; Yang, Z.G.; Wang, P.H.; Chen, J.M. Strain-induced refinement and thermal stability of a nanocrystalline steel produced by surface mechanical attritiontreatment. Mater. Sci. Eng. A 2013, 568, 176–183. [Google Scholar] [CrossRef]
- Botcharova, E.; Freudenberger, J.; Schultz, L. Mechanical and electrical properties of mechanically alloyed nanocrystalline Cu–Nb alloys. Acta Mater. 2006, 54, 3333–3341. [Google Scholar] [CrossRef]
- Freudenberger, J.; Botcharova, E.; Schultz, L. Formation of the microstructure in Cu-Nb alloys. J. Mater. Sci. 2004, 39, 5343–5345. [Google Scholar] [CrossRef]
- Benghalem, A.; Morris, D.G. Microstructure and mechanical properties of concentrated copper-niobium alloys prepared by mechanical alloying. Mater. Sci. Eng. A 1993, 161, 255–266. [Google Scholar] [CrossRef]
- Abad, M.D.; Parker, S.; Kiener, D.; Primorac, M.M.; Hosemann, P. Microstructure and mechanical properties of CuxNb1−x alloys prepared by ball milling and high pressure torsion compacting. J. Alloys Comp. 2015, 630, 117–125. [Google Scholar] [CrossRef]
- Lei, R.S.; Xu, S.Q.; Wang, M.P.; Wang, H.P. Microstructure and properties of nanocrystalline copper–niobium alloy with high strength and high conductivity. Mater. Sci. Eng. A 2013, 586, 367–373. [Google Scholar] [CrossRef]
- Botcharova, E.; Freudenberger, J.; Schultz, L. Cu–Nb alloys prepared by mechanical alloying and subsequent heat treatment. J. Alloys Comp. 2004, 365, 157–163. [Google Scholar] [CrossRef]
- Mula, S.; Bahmanpour, H.; Mal, S.; Kang, P.C.; Atwater, M.; Jian, W.; Scattergood, R.O.; Kochet, C.C. Thermodynamic feasibility of solid solubility extension of Nb in Cu and their thermal stability. Mater. Sci. Eng. A 2012, 539, 330–336. [Google Scholar] [CrossRef]
- Botcharova, E.; Heilmaier, M.; Freudenbergera, J.; Drew, G.; Kudashow, D.; Martin, U.; Schultz, L. Supersaturated solid solution of niobium in copper by mechanical alloying. J. Alloys Comp. 2003, 351, 119–125. [Google Scholar] [CrossRef]
- Lei, R.S.; Wang, M.P.; Li, Z.; Wei, H.G.; Yang, W.C.; Jia, Y.L.; Gong, S. Structure evolution and solid solubility extension of copper–niobium powders during mechanical alloying. Mater. Sci. Eng. A 2011, 528, 4475–4481. [Google Scholar] [CrossRef]
- Lei, R.S.; Wang, M.P.; Wang, H.P.; Xu, S.Q. New insights on the formation of supersaturated Cu-Nb solid solution prepared by mechanical alloying. Mater. Charact. 2016, 118, 324–331. [Google Scholar] [CrossRef]
- Botcharova, E.; Freudenberger, J.; Schultz, L. High thermal stability of mechanically-alloyed nanocrystalline Cu–Nb alloys. Int. J. Mater. Res. 2006, 97, 1350–1354. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The Kinetics of Precipitation from Supersaturated Solid Solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wagner, C. Theorie der Alterung von Niederschlägen durch Umlösen (Ostwald-Reifung). Z. Elektrochem. Beri. Bunsenges. Physikalische Chem. 1961, 65, 581–591. [Google Scholar]
- Barlow, I.C.; Jones, H.; Rainforth, W.M. The effect of heat treatment at 500–655 °C on the microstructure and properties of mechanically alloyed Al-Ti-O based material. Mater. Sci. Eng. A 2003, 351, 344–357. [Google Scholar] [CrossRef]
- Hansen, N.; Anderko, K. Constitution of Binary Alloys, 2nd ed.; McGraw-Hill: New York, NY, USA, 1958. [Google Scholar]
- Butrymowicz, D.B.; Manning, J.R.; Read, M.E. Diffusion Rate Data and Mass Transport Phenomena for Copper Systems, 1st ed.; International Copper Research Association: Washington, DC, USA, 1977. [Google Scholar]
- Ardell, A. On the coarsening of grain boundary precipitates. Acta Metall. 1972, 20, 601–619. [Google Scholar] [CrossRef]
- Gjostein, N.A. Diffusion, 1st ed.; American Society for Metals: Metals Park, OH, USA, 1973. [Google Scholar]
- Humphreys, F.J.; Hatherly, M. Recrystallisation and Related Annealing phenomena; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Fiedler, H.C. Grain oriented silicon- iron with a unique inhibition system for texture development. Metall. Trans. A 1977, 8, 1307–1312. [Google Scholar] [CrossRef]
- Fan, G.J.; Gao, W.N.; Quan, M.X.; Hu, Z.Q. Preparation and thermal stability of supersaturated nanocrystalline Al-Ti alloys. Mater. Lett. 1995, 23, 33–37. [Google Scholar] [CrossRef]
- Briant, C.L. Solid solubility and grain boundary segregation. Philos. Mag. Lett. 1996, 73, 345–349. [Google Scholar] [CrossRef]
- Frolov, T.; Darling, K.A.; Kecskes, L.J.; Mishin, Y. Stabilization and strengthening of nanocrystalline copper by alloying with tantalum. Acta Mater. 2012, 60, 2158–2168. [Google Scholar] [CrossRef]
- Hillert, M. On the theory of normal and abnormal grain growth. Acta Metall. 1965, 13, 227–238. [Google Scholar] [CrossRef]








| Preparation | 400 °C, 1 h | 700 °C, 1 h | 900 °C, 1 h | 900 °C, 3 h |
|---|---|---|---|---|
| ФNb,SEM (nm) | No | 78 | 175 | 207 |
| ФNb,TEM (nm) | No | 4 | 7 | 11 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, R.; Wang, M.; Xu, S.; Wang, H.; Chen, G. Microstructure, Hardness Evolution, and Thermal Stability Mechanism of Mechanical Alloyed Cu-Nb Alloy during Heat Treatment. Metals 2016, 6, 194. https://doi.org/10.3390/met6090194
Lei R, Wang M, Xu S, Wang H, Chen G. Microstructure, Hardness Evolution, and Thermal Stability Mechanism of Mechanical Alloyed Cu-Nb Alloy during Heat Treatment. Metals. 2016; 6(9):194. https://doi.org/10.3390/met6090194
Chicago/Turabian StyleLei, Ruoshan, Mingpu Wang, Shiqing Xu, Huanping Wang, and Guangrun Chen. 2016. "Microstructure, Hardness Evolution, and Thermal Stability Mechanism of Mechanical Alloyed Cu-Nb Alloy during Heat Treatment" Metals 6, no. 9: 194. https://doi.org/10.3390/met6090194
APA StyleLei, R., Wang, M., Xu, S., Wang, H., & Chen, G. (2016). Microstructure, Hardness Evolution, and Thermal Stability Mechanism of Mechanical Alloyed Cu-Nb Alloy during Heat Treatment. Metals, 6(9), 194. https://doi.org/10.3390/met6090194