High Temperature Strength and Hot Working Technology for As-Cast Mg–1Zn–1Ca (ZX11) Alloy
Abstract
:1. Introduction
2. Processing Map Methodology
3. Experimental Section
4. Results and Discussion
4.1. Microstructure of Cast Alloy
4.2. Ultimate Compressive Strength
4.3. Stress-Strain Behavior
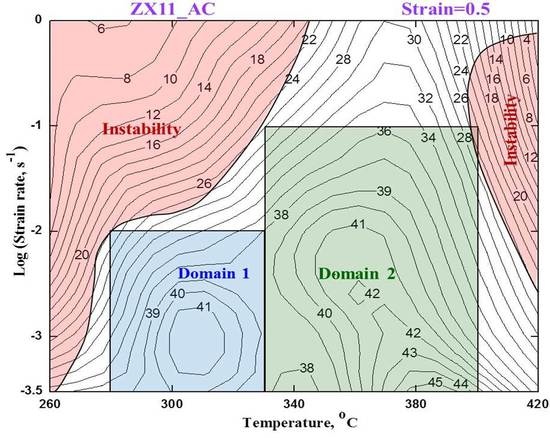
4.4. Processing Map
4.5. Activation Analysis
4.6. Flow Instability
5. Validation Experiments Using Forging
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Li, W.; Guan, S.; Chen, J.; Hu, J.; Chen, S.; Wang, L.; Zhu, S. Preparation and in vitro degradation of the composite coating with high adhesion strength on biodegradable Mg-Zn-Ca alloy. Mater. Charact. 2011, 62, 1158–1165. [Google Scholar] [CrossRef]
- Xu, Z.; Smith, C.; Chen, S.; Sankar, J. Development and microstructural characterization of Mg-Zn-Ca alloys for biomedical applications. Mater. Sci. Eng. B 2011, 176, 1660–1665. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and characterization of a new biomedical Mg-Zn-Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Akhtar, A.; Teghtsoonian, E. Substitutional solution hardening of magnesium single crystals. Philos. Mag. 1972, 25, 897–916. [Google Scholar] [CrossRef]
- Akhtar, A.; Teghtsoonian, E. Solid solution strengthening of magnesium single crystals—I, Alloying behaviour in basal slip. Acta Metall. 1969, 17, 1339–1349. [Google Scholar] [CrossRef]
- Akhtar, A.; Teghtsoonian, E. Solid solution strengthening of magnesium single crystals—II, The effect of solute on the ease of prismatic slip. Acta Metall. 1969, 17, 1351–1356. [Google Scholar] [CrossRef]
- Blake, A.H.; Cáceres, C.H. Solid Solution Effects on the Tensile Behavior of Concentrated Mg-Zn Alloys. In Essential Readings in Magnesium Technology; Mathaudhu, S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 263–267. [Google Scholar]
- Katsarou, L.; Suresh, K.; Rao, K.P.; Hort, N.; Blawert, C.; Mendis, C.L.; Dieringa, H. Microstructure and Properties of Magnesium Alloy Mg-1Zn-1Ca (ZX11). In Magnesium Technology 2015; Manuel, M.V., Singh, A., Alderman, M., Neelameggham, N.R., Eds.; TMS: Orlando, FL, USA, 2015; pp. 419–423. [Google Scholar]
- Nie, J.F.; Muddle, B.C. Precipitation hardening of Mg-Ca-(Zn) alloys. Scr. Mater. 1997, 37, 1475–1481. [Google Scholar] [CrossRef]
- Langelier, B.; Wang, X.; Esmaeili, S. Evolution of precipitation during non-isothermal ageing of an Mg-Ca-Zn alloy with high Ca content. Mater. Sci. Eng. A 2012, 538, 246–251. [Google Scholar] [CrossRef]
- Bamberger, M.; Levi, G.; Vander Sande, J.B. Precipitation hardening in Mg-Ca-Zn alloys. Metall. Mater. Trans. A 2006, 37, 481–487. [Google Scholar] [CrossRef]
- Hradilová, M.; Montheillet, F.; Fraczkiewicz, A.; Desrayaud, C.; Lejček, P. Effect of Ca-addition on dynamic recrystallization of Mg–Zn alloy during hot deformation. Mater. Sci. Eng. A 2013, 580, 217–226. [Google Scholar] [CrossRef]
- Tong, L.B.; Zheng, M.Y.; Cheng, L.R.; Kamado, S.; Zhang, H.J. Effect of extrusion ratio on microstructure, texture and mechanical properties of indirectly extruded Mg–Zn–Ca alloy. Mater. Sci. Eng. A 2013, 569, 48–53. [Google Scholar] [CrossRef]
- Tong, L.B.; Zheng, M.Y.; Zhang, D.P.; Gan, W.M.; Brokmeier, H.G.; Meng, J.; Zhang, H.J. Compressive deformation behavior of Mg–Zn–Ca alloy at elevated temperature. Mater. Sci. Eng. A 2013, 586, 71–77. [Google Scholar] [CrossRef]
- Li, W.J.; Deng, K.K.; Zhang, X.; Nie, K.B.; Xu, F.J. Effect of ultra-slow extrusion speed on the microstructure and mechanical properties of Mg-4Zn-0.5Ca alloy. Mater. Sci. Eng. A 2016, 677, 367–375. [Google Scholar] [CrossRef]
- Prasad, Y.V.R.K.; Rao, K.P.; Sasidhara, S. Hot Working Guide: A Compendium of Processing Maps, 2nd ed.; ASM International: Materials Park, OH, USA, 2015. [Google Scholar]
- Prasad, Y.V.R.K.; Seshacharyulu, T. Modelling of hot deformation for microstructural control. Inter. Mater. Rev. 1998, 43, 243–258. [Google Scholar] [CrossRef]
- Sneddon, I.N.; Hill, R.; Naghdi, P.M.; Ziegler, H. Progress in Solid Mechanics; Sneddon, I.N., Hill, R., Eds.; John Wiley: New York, NY, USA, 1965; Volume 4, pp. 91–193. [Google Scholar]
- Prasad, Y.V.R.K.; Rao, K.P. Processing maps and rate controlling mechanisms of hot deformation of electrolytic tough pitch copper in the temperature range 300–950 °C. Mater. Sci. Eng. A 2005, 391, 141–150. [Google Scholar] [CrossRef]
- Rao, K.P.; Prasad, Y.V.R.K. Materials modeling and finite element simulation of isothermal forging of electrolytic copper. Mater. Des. 2011, 32, 1851–1858. [Google Scholar]
- Suresh, K.; Rao, K.P.; Prasad, Y.V.R.K.; Hort, N.; Kainer, K.U. Effect of calcium addition on the hot working behavior of as-cast AZ31 magnesium alloy. Mater. Sci. Eng. A 2013, 588, 272–279. [Google Scholar] [CrossRef]
- Suresh, K.; Rao, K.P.; Prasad, Y.V.R.K.; Hort, N.; Kainer, K.U. Microstructure and mechanical properties of as-cast Mg-Sn-Ca alloys and effect of alloying elements. Trans. Nonferr. Met. Soc. China 2013, 23, 3604–3610. [Google Scholar] [CrossRef]
- Sastry, D.H.; Prasad, Y.V.R.K.; Vasu, K.I. On the stacking fault energies of some close-packed hexagonal metals. Scr. Metall. 1969, 3, 927–929. [Google Scholar] [CrossRef]
- Jonas, J.J.; Sellars, C.M.; Tegart, W.J.M. Strength and structure under hot working conditions. Metall. Rev. 1969, 14, 1–24. [Google Scholar]
- Frost, H.J.; Ashby, M.F. Deformation-Mechanism Maps; Pergamon Press: Oxford, UK, 1982; p. 44. [Google Scholar]
- Kobayashi, S.; Oh, S.I.; Altan, T. Metal Forming and the Finite-Element Method; Oxford University Press: Oxford, UK, 1989; pp. 1–41. [Google Scholar]
- Oh, S.I. Finite element analysis of metal forming processes with arbitrarily shaped dies. Int. J. Mech. Sci. 1982, 24, 479–493. [Google Scholar] [CrossRef]
















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, K.P.; Suresh, K.; Prasad, Y.V.R.K.; Dharmendra, C.; Hort, N.; Dieringa, H. High Temperature Strength and Hot Working Technology for As-Cast Mg–1Zn–1Ca (ZX11) Alloy. Metals 2017, 7, 405. https://doi.org/10.3390/met7100405
Rao KP, Suresh K, Prasad YVRK, Dharmendra C, Hort N, Dieringa H. High Temperature Strength and Hot Working Technology for As-Cast Mg–1Zn–1Ca (ZX11) Alloy. Metals. 2017; 7(10):405. https://doi.org/10.3390/met7100405
Chicago/Turabian StyleRao, Kamineni Pitcheswara, Kalidass Suresh, Yellapregada Venkata Rama Krishna Prasad, Chalasani Dharmendra, Norbert Hort, and Hajo Dieringa. 2017. "High Temperature Strength and Hot Working Technology for As-Cast Mg–1Zn–1Ca (ZX11) Alloy" Metals 7, no. 10: 405. https://doi.org/10.3390/met7100405
APA StyleRao, K. P., Suresh, K., Prasad, Y. V. R. K., Dharmendra, C., Hort, N., & Dieringa, H. (2017). High Temperature Strength and Hot Working Technology for As-Cast Mg–1Zn–1Ca (ZX11) Alloy. Metals, 7(10), 405. https://doi.org/10.3390/met7100405