The Corrosion Inhibition Effect of Triazinedithiol Inhibitors for Aluminum Alloy in a 1 M HCl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Weight Loss Test
2.3. Electrochemical Measurements
2.4. Scanning Electron Microscopy (SEM)
2.5. Quantum Chemical Calculation
3. Results
3.1. Weight Loss Study
3.1.1. Effect of Concentration
3.1.2. Effect of Temperature
3.2. Electrochemical Measurements
3.2.1 OCP Measurement
3.2.2. Potentiodynamic Polarization
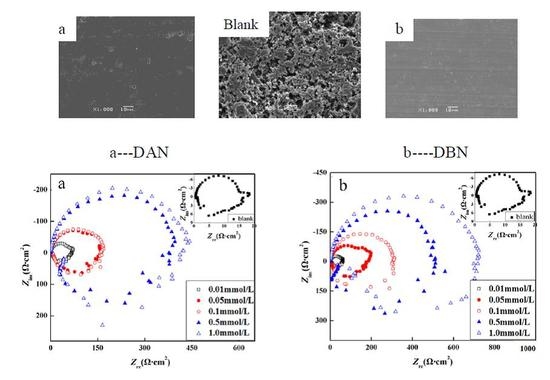
3.2.3. Electrochemical Impedance Spectroscopy (EIS)
3.3. Surface Morphology
3.4. Adsorption Isotherm
3.5. Quantum Chemical Calculation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dabalà, M.; Ramous, E.; Magrini, M. Corrosion resistance of cerium-based chemical conversion coatings on AA5083 aluminium alloy. Mater. Corros. 2004, 55, 381–386. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Daud, A.R.; Kamarudin, S.K. On the inhibition of mild steel corrosion by 4-amino-5-phenyl-4H-1,2,4-trizole-3-thiol. Corros. Sci. 2010, 52, 526–533. [Google Scholar] [CrossRef]
- Rosliza, R.; Nik, W.B.W.; Izman, S.; Prawoto, Y. Anti-corrosive properties of natural honey on Al–Mg–Si alloy in seawater. Curr. Appl. Phys. 2010, 10, 923–929. [Google Scholar] [CrossRef]
- Hill, J.A.; Markley, T.; Forsyth, M.; Howlett, P.C.; Hinton, B.R.W. Corrosion inhibition of 7000 series aluminium alloys with cerium diphenyl phosphate. J. Alloy. Compd. 2011, 509, 1683–1690. [Google Scholar] [CrossRef]
- Rosliza, R.; Nik, W.B.W.; Senin, H.B. The effect of inhibitor on the corrosion of aluminum alloys in acidic solutions. Mater. Chem. Phys. 2008, 107, 281–288. [Google Scholar] [CrossRef]
- Gudić, S.; Vrsalović, L.; Kliškić, M.; Jerković, I.; Radonić, A.; Zekić, M. Corrosion inhibition of AA5052 aluminium alloy in 0.5 M NaCl solution by different types of honey. Int. J. Electrochem. Sci. 2016, 11, 998–1011. [Google Scholar]
- Oguzie, E.E.; Onuoha, G.N.; Ejike, E.N. Effect of gongronema latifolium extract on aluminium corrosion in acidic and alkaline media. Pigment Resin Technol. 2007, 36, 44–49. [Google Scholar] [CrossRef]
- Al-Turkustani, A.M.; Arab, S.T.; Al-Dahiri, R.H. Aloe plant extract as environmentally friendly inhibitor on the corrosion of aluminum in hydrochloric acid in absence and presence of iodide ions. Mod. Appl. Sci. 2010, 4, 105–124. [Google Scholar] [CrossRef]
- Li, J.; Hurley, B.; Buchheit, R. Microelectrochemical characterization of the effect of rare earth inhibitors on the localized corrosion of AA2024-T3. J. Electrochem. Soc. 2015, 162, C563–C571. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Scully, J.R. Mass-transport-limited oxygen reduction reaction on AA2024-T3 and selected intermetallic compounds in chromate-containing solutions. Corrosion 2001, 57, 134–152. [Google Scholar] [CrossRef]
- Yurt, A.; Ulutas, S.; Dal, H. Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl. Surf. Sci. 2006, 253, 919–925. [Google Scholar] [CrossRef]
- Maayta, A.K.; Al-Rawashdeh, N.A.F. Inhibition of acidic corrosion of pure aluminum by some organic compounds. Corros. Sci. 2004, 46, 1129–1140. [Google Scholar] [CrossRef]
- Khaled, K.F. Electrochemical investigation and modeling of corrosion inhibition of aluminum in molar nitric acid using some sulphur-containing amines. Corros. Sci. 2010, 52, 2905–2916. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Hua, Y.X. Corrosion inhibition of aluminum in hydrochloric acid solution by alkylimidazolium ionic liquids. Mater. Chem. Phys. 2010, 119, 57–64. [Google Scholar] [CrossRef]
- Hachelef, H.; Benmoussat, A.; Khelifa, A.; Athmani, D.; Bouchareb, D. Study of corrosion inhibition by Electrochemical Impedance Spectroscopy method of 5083 aluminum alloy in 1 M HCl solution containing propolis extract. J. Mater. Environ. Sci. 2016, 7, 1751–1758. [Google Scholar]
- Zhao, Q.; Tang, T.; Dang, P.; Zhang, Z.; Wang, F. Preparation and analysis of complex barrier layer of heterocyclic and long-chain organosilane on copper alloy surface. Metals 2016, 6, 162. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kim, Y.W.; Chung, K.; Kim, N.K.; Kim, J.S. Corrosion Inhibition Properties of Triazine Derivatives Containing Carboxylic Acid and Amine Groups in 1 M HCl Solution. Ind. Eng. Chem. Res. 2013, 52, 10880–10889. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Zhang, H.; Wang, F. Study on triazinethiol electropolymerized films prepared by cyclic voltammetry and galvanostatic on copper alloy surface. Int. J. Electrochem. Sci. 2011, 6, 4404–4410. [Google Scholar]
- Wang, F.; Liu, J.; Li, Y.; Fan, R. Complex barrier layer of triazinedithoil prepared by electrodeposition and initiated polymerization on aluminum alloy towards corrosion protection. Int. J. Electrochem. Sci. 2012, 7, 3672–3680. [Google Scholar]
- Shalabi, K.; Abdallah, Y.M.; Fouda, A.S. Corrosion inhibition of aluminum in 0.5 M HCl solutions containing phenyl sulfonylacetophenoneazo derivatives. Res. Chem. Intermed. 2014, 41, 4687–4711. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.O.; Obot, I.B. Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corrosion in H2SO4. Corros. Sci. 2011, 53, 263–275. [Google Scholar] [CrossRef]
- Eddy, N.O.; Momoh-Yahaya, H.; Oguzie, E.E. Theoretical and experimental studies on the corrosion inhibition potentials of some purines for aluminum in 0.1 M HCl. J. Adv. Res. 2015, 6, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Halambek, J.; Jukić, M.; Berković, K.; Vorkapić-Furač, J. Investigation of novel heterocyclic compounds as inhibitors of Al-3Mg alloy corrosion in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2012, 7, 1580–1601. [Google Scholar]
- Schorr, M.; Yahalom, J. The significance of the energy of activation for the dissolution reaction of metal in acids. Corros. Sci. 1972, 12, 867–868. [Google Scholar] [CrossRef]
- Kabir, K.B.; Mahmud, I. Study of erosion-corrosion of stainless steel, brass and aluminum by open circuit potential measurements. J. Chem. Eng. 2010, 25, 13–17. [Google Scholar] [CrossRef]
- Abiola, O.K.; Otaigbe, J.O.E. Effect of common water contaminants on the corrosion of aluminium alloys in ethylene glycol–water solution. Corros. Sci. 2008, 50, 242–247. [Google Scholar] [CrossRef]
- Maghraby, A.A.E. Corrosion inhibition of aluminum in hydrochloric acid solution using potassium iodate inhibitor. Open Corros. J. 2009, 2, 189–196. [Google Scholar] [CrossRef]
- Ladha, D.G.; Wadhwani, P.M.; Kumar, S.; Shah, N.K. Evaluation of corrosion inhibitive properties of trigonellafoenum-graecum for pure aluminium in hydrochloric acid. J. Mater. Environ. Sci. 2015, 6, 1200–1207. [Google Scholar]
- Şafak, S.; Duran, B.; Yurt, A.; Türkoğlu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 54, 251–259. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Fouda, A.S.; Mohamed, F.S.; El-Sherbeni, M.W. Corrosion inhibition of aluminum–silicon alloy in hydrochloric acid solutions using carbamidic thioanhydride derivatives. J. Bio- Tribo-Corros. 2016, 2, 1–16. [Google Scholar] [CrossRef]
- Kathirvel, K.; Thirumalairaj, B.; Jaganathan, M. Quantum chemical studies on the corrosion inhibition of mild steel by piperidin-4-one derivatives in 1 M H3PO4. Open J. Met. 2014, 4, 73–85. [Google Scholar] [CrossRef]








| Conc. | DAN | DBN | ||
|---|---|---|---|---|
| (mM) | CR (g·m−2·h−1) | ηw (%) | CR (g·m−2·h−1) | ηw (%) |
| Blank | 90.90 | 82.15 | ||
| 0.01 | 20.00 | 78.41 | 46.18 | 43.79 |
| 0.03 | 7.57 | 91.60 | 12.94 | 84.25 |
| 0.05 | 4.98 | 94.56 | 3.40 | 95.86 |
| 0.10 | 4.04 | 95.56 | 2.09 | 97.46 |
| 0.50 | 1.60 | 98.24 | 1.10 | 98.66 |
| 1.00 | 1.31 | 98.56 | 0.65 | 99.21 |
| Inhibitor | C (mM) | Ecorr (mV/SCE) | Icorr (μA·cm−2) | βa (mV·dec−1) | βc (mV·dec−1) | ηp (%) |
|---|---|---|---|---|---|---|
| Blank | −735.90 | 2852.40 | 118.30 | 90.50 | ||
| DAN | 0.01 | −742.10 | 835.30 | 128.30 | 88.70 | 70.72 |
| 0.05 | −739.00 | 316.50 | 136.50 | 96.40 | 88.90 | |
| 0.10 | −740.40 | 251.90 | 140.10 | 90.80 | 90.17 | |
| 0.50 | −742.10 | 145.60 | 143.30 | 93.90 | 94.90 | |
| 1.00 | −742.90 | 63.60 | 147.40 | 107.50 | 97.77 | |
| DBN | 0.01 | −740.40 | 1198.40 | 121.20 | 101.20 | 57.99 |
| 0.05 | −741.20 | 468.50 | 128.90 | 98.40 | 83.58 | |
| 0.10 | −741.40 | 166.80 | 136.30 | 96.30 | 95.91 | |
| 0.50 | −746.50 | 52.70 | 148.30 | 97.60 | 98.15 | |
| 1.00 | −746.80 | 33.50 | 143.00 | 104.20 | 98.83 |
| Inhibitor | T (°C) | Kads (L·mol−1) | ΔGθ (kJ·mol−1) |
|---|---|---|---|
| DAN | 30 °C | 370,370 | −42.4 |
| 35 °C | 136,990 | −40.6 | |
| DBN | 30 °C | 169,490 | −40.5 |
| 35 °C | 22,830 | −36.0 |
| Compound | EHOMO (eV) | ELUMO (eV) | ΔEgap (eV) |
|---|---|---|---|
| DAN | −3.456 | −0.789 | 2.667 |
| DBN | −2.748 | −0.844 | 1.904 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Tang, T.; Dang, P.; Zhang, Z.; Wang, F. The Corrosion Inhibition Effect of Triazinedithiol Inhibitors for Aluminum Alloy in a 1 M HCl Solution. Metals 2017, 7, 44. https://doi.org/10.3390/met7020044
Zhao Q, Tang T, Dang P, Zhang Z, Wang F. The Corrosion Inhibition Effect of Triazinedithiol Inhibitors for Aluminum Alloy in a 1 M HCl Solution. Metals. 2017; 7(2):44. https://doi.org/10.3390/met7020044
Chicago/Turabian StyleZhao, Qian, Tiantian Tang, Peilin Dang, Zhiyi Zhang, and Fang Wang. 2017. "The Corrosion Inhibition Effect of Triazinedithiol Inhibitors for Aluminum Alloy in a 1 M HCl Solution" Metals 7, no. 2: 44. https://doi.org/10.3390/met7020044
APA StyleZhao, Q., Tang, T., Dang, P., Zhang, Z., & Wang, F. (2017). The Corrosion Inhibition Effect of Triazinedithiol Inhibitors for Aluminum Alloy in a 1 M HCl Solution. Metals, 7(2), 44. https://doi.org/10.3390/met7020044