Surface Characterization and Corrosion Resistance of 36Cr-Ni-Mo4 Steel Coated by WC-Co Cermet Electrode Using Micro-Electro Welding
Abstract
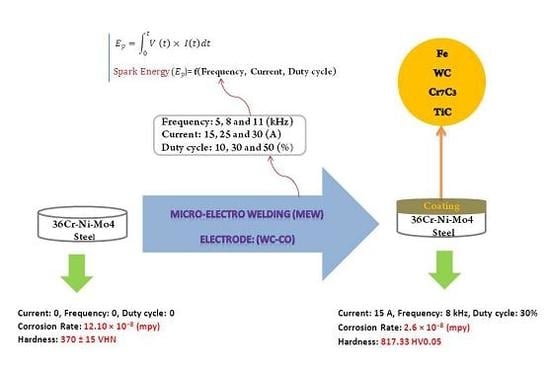
:1. Introduction
2. Material and Methods
3. Calculations
3.1. Spark Energy Calculation
3.2. Coating Efficiency Calculation
4. Results and Discussion
4.1. Microstructure Analysis
4.2. Surface and Cross Section Studies
4.3. Microhardness Profile
4.4. Microroughness Profile
4.5. Results of Corrosion Test
5. Conclusions
- With increasing current limit, frequency and duty cycle of the process, spark energy increases.
- As spark energy increases, efficiency of coating increases to 80% and then decreases. If deposition efficiency is more than 50% the process will be high in efficiency, thus, in the energy range of 0.5–3.5 mJ the process will have the appropriate efficiency.
- Metallographic studies indicate that the microstructure of the substrate is composed of pearlite and bainite with a hardness of 370 ± 15 VHN.
- As spark energy increases up to 2.17 mJ, thickness of coating increases to 8.31 μm and then decreases.
- The results of the EDS analysis indicate that with increasing spark energy, the amount of Tungsten in the surface increases and then decreases. At first, a small amount of Tungsten (17.3 wt %) can be seen on the surface. As spark energy increases, the amount of Tungsten in the surface increases to 41.95 wt %. At higher spark energy, the amount of Tungsten in the surface is reduced.
- SEM observations demonstrate that concentrations of Tungsten and Iron are at a maximum limit up to an approximate distance of 6 μm from the surface, but at a distance of 6–8 μm from the surface Iron concentration has increased progressively.
- Obtained peaks of XRD analysis confirmed that in addition to metallic Iron phase with BCC structure, peaks related to phases Tungsten Carbide, Cr7C3 and Titanium Carbide also can be observed.
- Surface hardness increases to 817.33 HV0.05 with spark energy increasing up to 1.03 mJ, and then reducing.
- With increasing spark energy the surface roughness increases.
- Maximum corrosion rate (12.1 × 10−8 mpy) relates to the reference sample and minimum corrosion rate (2.6 × 10−8 mpy) relates to the sample with maximum coating efficiency (AFD150830).
Author Contributions
Conflicts of Interest
References
- Vergne, C.; Boher, C.; Gras, R. Analysis of the friction and wear behavior of hot work tool scale: Application to the hot rolling process. Wear 2001, 250, 322–333. [Google Scholar] [CrossRef]
- Barrau, O.; Boher, C.; Gras, R.; Rezai-Aria, F. Analysis of the friction and wear behavior of hot work tool steel for forging. Wear 2003, 255, 1444–1454. [Google Scholar] [CrossRef]
- Podgornik, B.; Hogmark, S.; Sandberg, O.; Leskovsek, V. Wear resistance and anti-sticking properties of duplex treated forming tool steel. Wear 2003, 254, 1113–1121. [Google Scholar] [CrossRef]
- Alsaran, A.; Yildiz, F.; Celik, A. Effects of post-aging on wear and corrosion properties of nitrided AISI 4140 steel. Surf. Coat. Technol. 2006, 201, 3147–3154. [Google Scholar] [CrossRef]
- De Souza Brandolt, C.; Noronha, L.C.; Hidalgo, G.E.N.; Takimi, A.S.; Schroeder, R.M.; De Fraga Malfatti, C. Niobium coating applied by HVOF as protection against hydrogen embrittlement of API 5CT P110 steel. Surf. Coat. Technol. 2017, 322, 10–18. [Google Scholar] [CrossRef]
- Rao, Z.; O’Connor, B.H.; Williams, J.S.; Sood, D.K. Carbon implantation into hard Chromium coatings. Surf. Coat. Technol. 1996, 84, 512–518. [Google Scholar] [CrossRef]
- Walter, K.C.; Scheuer, J.T.; McIntyre, P.C.; Kodali, P.; Yu, N.; Nastasi, M. Increased wear resistance of electrodeposited chromium through applications of plasma source ion implantation techniques. Surf. Coat. Technol. 1996, 85, 1–6. [Google Scholar] [CrossRef]
- Kessler, O.H.; Hoffmann, F.T.; Mayr, P. Combinations of coating and heat treating processes: Establishing a system for combined processes and examples. Surf. Coat. Technol. 1998, 108–109, 211–216. [Google Scholar] [CrossRef]
- Podgornik, B.; Vizintin, J.; Wanstrand, O.; Larsson, M.; Hogmark, S. Wear and friction behaviour of duplex-treated AISI 4140 steel. Surf. Coat. Technol. 1999, 120–121, 502–508. [Google Scholar] [CrossRef]
- Podgornik, B. Coated machine elements—Fiction or reality. Surf. Coat. Technol. 2001, 146–147, 318–323. [Google Scholar] [CrossRef]
- Podgornik, B.; Vizintin, J. Rolling contact properties of ta-C coated low alloy steel. Surf. Coat. Technol. 2002, 157, 257–261. [Google Scholar] [CrossRef]
- Han, S.; Lin, J.H.; Tsai, S.H.; Chung, S.C.; Wang, D.Y.; Lu, F.H.; Shih, H.C. Corrosion and tribological studies of chromium nitride coated on steel with an interlayer of electroplated chromium. Surf. Coat. Technol. 2000, 133–134, 460–465. [Google Scholar] [CrossRef]
- Chiu, L.H.; Yang, C.F.; Hsieh, W.C.; Cheng, A.S. Effect of contact pressure on wear resistance of AISI H13 tool steels with chromium nitride and hard chromium coatings. Surf. Coat. Technol. 2002, 154, 282–288. [Google Scholar] [CrossRef]
- Chiu, L.H.; Yang, C.F.; Liu, P.M. Wear resistance of JIS SKD61 tool steels with Cr based coatings. Surf. Eng. 2000, 16, 257–261. [Google Scholar] [CrossRef]
- Hurkmans, T.; Lewis, D.D.; Brooks, J.S.; Munz, W.D. Chromium nitride coatings grown by unbalanced magnetron (UBM) and combined arc/unbalanced magnetron (ABS™) deposition techniques. Surf. Coat. Technol. 1996, 86–87, 192–199. [Google Scholar] [CrossRef]
- Johnson, R.N.; Sheldon, G.L. Advances in the electrospark deposition coating process. J. Vac. Sci. Technol. 1986, 4, 2740–2746. [Google Scholar] [CrossRef]
- Grun, R. Combination of different plasma assisted processes with pulsed D.C.: Cleaning, nitriding and hardcoatings. Surf. Coat. Technol. 1995, 74–75, 598–603. [Google Scholar] [CrossRef]
- Manjunatha, M.; Kkulkarni, R.S.; Krishna, M. Investigation of HVOF thermal sprayed Cr3C2-NiCr cermet carbide coatings on erosive performance of AISI 316 stainless steel. Procedia Mater. Sci. 2014, 5, 622–629. [Google Scholar] [CrossRef]
- Algodi, S.J.; Murray, J.W.; Fay, M.W.; Clare, A.T.; Brown, P.D. Electrical discharge coating of nanostructured TiC-Fe cermets on 304 stainless Steel. Surf. Coat. Technol. 2016, 307, 639–649. [Google Scholar] [CrossRef]
- Espallargas, N.; Berget, J.; Guilemany, J.M.; Benedetti, A.V.; Suegama, P.H. Cr3C2–NiCr and WC–Ni thermal spray coatings as alternatives to hard chromium for erosion–corrosion resistance. Surf. Coat. Technol. 2008, 202, 1405–1417. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Holdren, R.L.; Brown, L.E. Electro-Spark Deposition. Adv. Mater. Process 2003, 161, 35–37. [Google Scholar]
- Johnson, R.N. ElectroSpark Deposition: Principles and Applications. In Proceedings of the 45th Annual Technical Conference of the Society of Vacuum Coaters, Richland, WA, USA, 13–18 April 2002; pp. 87–92. [Google Scholar]
- Welsh, N.C.; Watts, P.E. Spark Hardening of Cutting Tools: Austenite Formation and Edge Erosion. J. Iron Steel Inst. 1962, 5, 333. [Google Scholar]
- Schuh, C.A.; Nieh, T.G.; Yamasaki, T. Hall-Petch breakdown manifested in abrasive wear resistance of nanocrystalline nickel. Scr. Mater. 2002, 46, 735–740. [Google Scholar] [CrossRef]
- Banovic, S.W.; DuPont, J.N.; Marder, A.R. Iron Aluminide Weld Overlay Coatings for Bolier Tube Protection in Coal-Fried Low Nox Boilers. In Proceedings of the 11th Annual Conference on Fossil Energy Materials, Knoxville, TN, USA, 20–22 May 1997; p. 279. [Google Scholar]
- Polyachenko, A.U. New methods of Electrical Working of Materials. Mashgiz Moscow 1955, 46, 352. [Google Scholar]
- Gould, J.E. Welding Handbook, 9th ed.; American Welding Society: Miami, FL, USA, 2007; Volume 3, pp. 598–602. [Google Scholar]
- Wei, X.; Chen, Z.; Zhong, J.; Xiang, Y. Feasibility of preparing Mo2FeB2-based cermet coating by electrospark deposition on high speed steel. Surf. Coat. Technol. 2016, 296, 58–64. [Google Scholar] [CrossRef]
- Korkmaz, K. Investigation and characterization of electrospark deposited chromium carbide-based coating on the steel. Surf. Coat. Technol. 2015, 272, 1–7. [Google Scholar] [CrossRef]
- Radek, N.; Konstanty, J. Cermet ESD Coatings Modified by LaserTreatment. Arch. Metall. Mater. 2012, 57, 665–670. [Google Scholar] [CrossRef]
- Tang, C.-B.; Lin, D.-X.; Wang, Z.; Gao, Y. Electro-spark alloying using graphite electrode on titanium alloy surface for biomedical applications. Appl. Surf. Sci. 2011, 257, 6364–6371. [Google Scholar]
- Frangini, S.; Masci, A.; Di Bartolomeo, A. Cr7C3-based cermet coating deposited on stainless steel by electrospark process: Structural characteristics and corrosion behavior. Surf. Coat. Technol. 2002, 149, 279–286. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Y. Surface modification of resistance welding electrode by electrospark deposited composite coatings Part II. Metallurgical behavior during welding. Surf. Coat. Technol. 2006, 201, 2419–2430. [Google Scholar] [CrossRef]
- Frangini, S.; Masci, A. A study on the effect of a dynamic contact force control for improving electrospark coating properties. Surf. Coat. Technol. 2010, 204, 2613–2623. [Google Scholar] [CrossRef]
- Steel VCMO230 (Mat. No. 1.7707, DIN 30CrMoV9, AISI 4340). Heat Treatment Manual; Data Sheet, SIJ Metal Ravne Co.: Carinthia, Slovenia, 2005. [Google Scholar]
- Soltanieh, M.; Aghajani, H.; Mahboubi, F.; Nekouee, K.A. Surface characterization of multiple coated H11 hot work steel by plasma nitriding and hard chromium electroplating processes. Vacuum 2012, 86, 1470–1476. [Google Scholar] [CrossRef]
- Gaskell, D.R. Introduction to the Thermodynamics of Materials; Taylor & Francis: New York, NY, USA, 1995. [Google Scholar]
- Mo, D.F.; Hu, Z.F.; Chen, S.J.; Wang, C.X.; He, G.Q. Microstructure and hardness of T250 Maraging steel in heat affected zone. J. Iron Steel Res. Int. 2009, 16, 87–91. [Google Scholar] [CrossRef]
- Gharibshahiyan, E.; Raouf, A.H.; Parvin, N.; Rahimian, M. The effect of microstructure on hardness and toughness of low carbon welded steel using inert gas welding. Mater. Des. 2011, 32, 2042–2048. [Google Scholar] [CrossRef]
- Li, L.C.; Chai, M.Y.; Li, Y.Q.; Bai, W.J.; Duan, Q. Effect of Welding Heat Input on Grain Size and Microstructure of 316L Stainless Steel Welded Joint. Mech. Mater. 2013, 331, 578–582. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Shu, F.; He, W.; Wang, G. Microstructure and Corrosion Behavior of Simulated Welding HAZ of Q315NS Steel in Sulfuric Acid Solution. Metals 2017, 7, 194. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Li, G.; Han, P.; Liang, W. Characterization of the Microstructure, Mechanical Properties, and Corrosion Resistance of a Friction-Stir-Welded Joint of Hyper Duplex Stainless Steel. Metals 2017, 7, 138. [Google Scholar] [CrossRef]
- Yingsamphancharoen, T.; Srisuwan, N.; Rodchanarowan, A. The Electrochemical Investigation of the Corrosion Rates of Welded Pipe ASTM A106 Grade B. Metals 2016, 6, 207. [Google Scholar] [CrossRef]
- Geng, X.; Feng, H.; Jiang, Z.; Li, H.; Zhang, B.; Zhang, S.; Wang, Q.; Li, J. Microstructure, Mechanical and Corrosion Properties of Friction StirWelding High Nitrogen Martensitic Stainless Steel 30Cr15Mo1N. Metals 2016, 6, 301. [Google Scholar] [CrossRef]
- Mohammed, G.R.; Ishak, M.; Aqida, S.N.; Abdulhadi, H.A. Effects of Heat Input on Microstructure, Corrosion and Mechanical Characteristics of Welded Austenitic and Duplex Stainless Steels: A Review. Metals 2017, 7, 39. [Google Scholar] [CrossRef]
















| Element | C | Si | Cr | Mo | V | Ni | Mn | S | P |
|---|---|---|---|---|---|---|---|---|---|
| Wt % | 0.3 | 0.25 | 2.9 | 0.22 | 0.18 | 0.1 | 0.54 | 0.004 | 0.003 |
| Duty Cycle (%) | Frequency (kHz) | Current (A) | Sample Code |
|---|---|---|---|
| 10 | 5 | 15 | AFD150510 |
| 25 | AFD250510 | ||
| 35 | AFD350510 | ||
| 8 | 15 | AFD150810 | |
| 25 | AFD250810 | ||
| 35 | AFD350810 | ||
| 11 | 15 | AFD151110 | |
| 25 | AFD251110 | ||
| 35 | AFD351110 | ||
| 30 | 5 | 15 | AFD150530 |
| 25 | AFD250530 | ||
| 35 | AFD350530 | ||
| 8 | 15 | AFD150830 | |
| 25 | AFD250830 | ||
| 35 | AFD350830 | ||
| 11 | 15 | AFD151130 | |
| 25 | AFD251130 | ||
| 35 | AFD351130 | ||
| 50 | 5 | 15 | AFD150550 |
| 25 | AFD250550 | ||
| 35 | AFD350550 | ||
| 8 | 15 | AFD150850 | |
| 25 | AFD250850 | ||
| 35 | AFD350850 | ||
| 11 | 15 | AFD151150 | |
| 25 | AFD251150 | ||
| 35 | AFD351150 |
| Element | Series | AFD150510 | AFD351150 | AFD250550 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unn. c | Norm. c | Atom. c | Unn. c | Norm. c | Atom. c | Unn. c | Norm. c | Atom. c | ||
| (wt %) | (wt %) | (at %) | (wt %) | (wt %) | (at %) | (wt %) | (wt %) | (at %) | ||
| Carbon | K series | 3.12 | 3.6 | 17.68 | - | - | - | 8.29 | 10.7 | 45.34 |
| Titanium | K series | 1.08 | 1.25 | 1.54 | - | - | - | 0.96 | 1.24 | 1.31 |
| Chromium | K series | 6.27 | 7.24 | 8.21 | - | - | - | - | - | - |
| Manganese | K series | 0.72 | 0.83 | 0.89 | - | - | - | - | - | - |
| Iron | K series | 57 | 61.98 | 54.9 | 59.69 | 67.17 | 84.18 | 30.85 | 39.81 | 36.28 |
| Cobalt | K series | 2.52 | 2.91 | 2.91 | 2.15 | 2.42 | 2.87 | 3.02 | 3.9 | 3.37 |
| Nickel | K series | 4.47 | 5.16 | 5.19 | 1.49 | 1.68 | 2 | 1.86 | 2.4 | 2.08 |
| Tungsten | L series | 15.4 | 17.03 | 8.67 | 25.54 | 28.74 | 10.94 | 32.5 | 41.95 | 11.61 |
| Sample | Corrosion Current Density (µA·cm−2) | Corrosion Potential (E vs. SCE) | βa (V/decade) | βc (V/decade) | Rp (ohm) | Corrosion Rate (mpy) × 10−8 |
|---|---|---|---|---|---|---|
| AFD150830 | 1.31 | −0.53 | 0.0143 | 0.0326 | 3397 | 2.6 |
| AFD250550 | 1.58 | −0.58 | 0.0444 | 0.2445 | 10,362 | 3.14 |
| AFD351150 | 2.13 | −0.56 | 0.0311 | 0.2350 | 5598 | 4.23 |
| AFD150510 | 2.51 | −0.54 | 0.0388 | 0.0776 | 4474 | 4.99 |
| Reference | 6.02 | −0.61 | 0.1612 | 0.0424 | 2421 | 12.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhideh, M.; Aghajani, H.; Pourbagheri, H. Surface Characterization and Corrosion Resistance of 36Cr-Ni-Mo4 Steel Coated by WC-Co Cermet Electrode Using Micro-Electro Welding. Metals 2017, 7, 308. https://doi.org/10.3390/met7080308
Azhideh M, Aghajani H, Pourbagheri H. Surface Characterization and Corrosion Resistance of 36Cr-Ni-Mo4 Steel Coated by WC-Co Cermet Electrode Using Micro-Electro Welding. Metals. 2017; 7(8):308. https://doi.org/10.3390/met7080308
Chicago/Turabian StyleAzhideh, Mohamad, Hossein Aghajani, and Hadi Pourbagheri. 2017. "Surface Characterization and Corrosion Resistance of 36Cr-Ni-Mo4 Steel Coated by WC-Co Cermet Electrode Using Micro-Electro Welding" Metals 7, no. 8: 308. https://doi.org/10.3390/met7080308
APA StyleAzhideh, M., Aghajani, H., & Pourbagheri, H. (2017). Surface Characterization and Corrosion Resistance of 36Cr-Ni-Mo4 Steel Coated by WC-Co Cermet Electrode Using Micro-Electro Welding. Metals, 7(8), 308. https://doi.org/10.3390/met7080308