2. Experimental Procedure
During this work, two main sets of data were collected—one during the production trials of forty semi-finished extruded products, and the other one through laboratory analysis, performed to assess their quality.
The process parameters were monitored by process-control software, which manages data about material flow speed, temperature and the liquid nitrogen valve opening. In detail, data about dye temperature was collected through four thermocouples (TC) installed within the mold (
Figure 3) and two laser pyrometers.
The thermocouples used in this work were Chromel/Alumel (K) thermocouples with mineral oxide insulation and Inconel 600 as the sheath material. The sheath had a 3.2 mm diameter and 2000 mm length. The laser pyrometer temperature method measured the emitted thermal radiation, which directly corresponded to the temperature and surface emissivity of the target. In detail, the pyrometer sensors detected the amount of infrared radiation emitted by the measured object. The pyrometers were installed in front of the exit of the extrusion dye (
Figure 4).
On the other hand, the surface defects of the extruded semi-products were characterized in the junction zone of the billets, since this is an accumulation area for the defects [
1,
2]. The samples were drawn from the extruded semi-products and polished following the common standards. The chemical compositions of the samples were acquired through a scanning electron microscope (SEM) (Carl Zeiss AG, Oberkochen, Germany) Zeiss EVO 50
®, using a back-scattered electron (SEM-BSE) (INCA Oxford Instrument, Oxford, UK) detector and energy dispersive X-ray microanalysis (SEM-EDS) (INCA Oxford Instrument, Oxford, UK). In addition, the same instrument was exploited to collect morphological images through a secondary electron detector (SEM-SE) (INCA Oxford Instrument, Oxford, UK).
The samples were etched electrochemically using a Barker’s reagent. The electrolytic solution was prepared by mixing 40 parts of water (H2O) for each volume-part of tetrafluoboric acid (HBF4). The electrochemical anodization was performed by applying a current density of 0.2 A/cm2 (20 V dc) for 40–80 s.
The metallographic analysis was executed using a cross polarized incident light. Afterwards, the index used to evaluate the average grain dimensions was calculated via the Heyn intercept method, following the ASTM E112-13 standard.
Roughness measurements were performed on the surface of the body of the extruded semi-product, hence, not in the junction area. Profile roughness measurements were taken perpendicularly to the extrusion direction, using a stylus Mahr PGK MFK-250® (Mahr GmbH, Göttingen, Germany) tester with a tip radius of 2 μm and a vertical measure range of +/−250 μm. The data were collected following the standard, UNI EN ISO 4288-2000. The translational speed of the measurement was 0.5 mm/s along an exploration length of 5.6 mm. The base length was 0.8 mm and the total evaluated length was 4.0 mm. The collected data were filtered using a Gaussian filter with a wavelength cutter of 0.8 mm.
Surface texture measurements were performed using an Alicona InfiniteFocus® (Alicona Imaging GmbH, Graz, Austria) microscope, which exploits focus-variation technology. The data were acquired in a similar position to those collected for the profile roughness measurements. The surface texture measurements parameters are listed as follows: the total evaluated area measured 4.0 × 4.0 mm and the base length was 0.8 mm. The collected data were filtered again using a Gaussian filter with a wavelength cutter of 0.8 mm.
3. Results
The data collected during the extrusion trials are summarized in
Table 1. This sets out the data regarding the most meaningful specimens that were analyzed in this study. It should be underlined that the samples numbered from 1 to 15 were processed through a conventional extrusion, and then, the liquid nitrogen valve was opened and the specimens numbered up to 25 were subjected to transient conditions. Thus, only the semi-products numbered from 26 to 40 were extruded with the liquid nitrogen mold cooling system working at full capacity.
The data regarding the characterization of defects are reported below. Two different species of defects were detected on the surface in the junction area of the extruded semi-products. Their SEM-BSE images are shown in
Figure 5, and the acquired chemical compositions are quoted in
Table 2.
Therefore, the analyzed defects can be classified as pick-up and dye pick-up. Pick-ups (
Figure 5a—point C) are intermittent score lines of varying lengths, which end in a fleck of aluminum debris. They can be easily identified by their chemical composition, which is the same as that of the extruded aluminum alloy [
23]. The exogenous deposited layers were composed by a different material and they can be recognized as dye pick-up (
Figure 5b—point A,
Figure 5a—point A), which are particles worn-out of the extrusion dye and deposited on the semi-product surface [
23,
24,
25,
26,
27].
Thereafter, the products, subjected to the whole production cycle, including painting and chromium plating, were also analyzed. The typical section, recorded via SEM-BSE, of the surface defects after these processes, is shown in
Figure 6. It is worth highlighting that the paint layer does not follow the defect profile; it would thus cover and hide the defects featured by heights up to 15 μm.
Through optical microscopy, a metallographic analysis was performed on the tested samples.
Figure 7 sets out two micrographs obtained after threating the samples via Barker electrochemical etching. However, this analysis did not highlight any detail about the microstructure, which could be relevant or have influence on the generation of defects. Indeed, at the grain boundaries no secondary phase or trace of segregation phenomena is present [
28].
Moreover, the microstructures appeared homogeneous across the junction area sections (
Figure 8a). Thus, no relationship between the microstructure and the surface defects was identified. Moreover, no differences in the morphologies of the crystal grains were detected; all the junction areas of the tested specimens featured coarse equiaxial microstructures, without been affected by any variations in the working parameters.
However, few millimeters away from the junction areas, the microstructures of the specimens were very different (
Figure 8b). The grains displayed a lamellar structure, oriented along the extrusion direction, usually featuring the extruded semi-products. This occurred because the process arrest leaves the junction area for a much longer time in contact with the mold after being deformed. Thus, a recrystallization phenomenon was promoted, as testified by the loss of the extrusion deformation pattern of the grains [
29].
Moreover, the grain size number G, an index measuring the average grain dimensions, was calculated through the Heyn intercept method and the results are reported in
Table 3.
Furthermore, a SEM-SE morphological analysis wasperformed, to qualitatively assess the differences between the different mold cooling methodologies. The most interesting results concerned billets extruded with the same working parameters. In
Figure 9, the junction areas of billets 5-2 (air cooled dye) and 25-4 (liquid nitrogen cooled die) are displayed in detail. Compared to the 25-4 sample, the 5-2 specimen showed much larger defects and a large quantity of the material adhered onto the surface.
The results of the roughness tests, performed on the surface of the body of the extruded semi-product—hence not in the junction areas—are shown in
Table 4. The mean roughness grew with the mold cooling liquid nitrogen valve aperture during the extrusion trials. On the other hand, the measured maximum roughness displayed a decreasing trend under the same operative conditions.
The surface texture data, collected via microscopy focus-variation technology, are set out below—both the parameters describing the roughness (
Table 5) and the height histogram of the surfaces and its statistics (
Figure 10 and
Table 6). The results are similar to those for the profile roughness. The mean values of the surface roughness slightly increased with use of the liquid nitrogen cooling system. On the other hand, the parameters describing the maxima and the minima of the surface, underwent a decreasing trend with the use of the liquid nitrogen cooling system.
In detail, the skewness values indicated that the height distribution of the liquid nitrogen-cooled sample was more “valley-tailed” than the traditionally extruded surface. For both distributions, the kurtosis parameter highlighted a leptokurtic behavior with a positive excess kurtosis. The higher value of the sample traditionally extruded testifies the presence of larger tails in its height distribution, due to the occurrence of larger surface defects in the sample produced through this technology, as observed via other characterization techniques as well.
4. Discussion
The experimental data from the current study testify that the liquid nitrogen cooling effect is not instantaneous and a transition time is required before a steady working regime temperature can be reached and maintained. This is related to the mold’s thermal inertia. Furthermore, when using this technology, the working temperature becomes lower relative to conventional air cooling of the extrusion mold. However, the differences between temperatures measured at the different dye holes broaden. This phenomenon may be related to the inhomogeneity of the heat removal, linked to the non-optimal sizing of the cooling channel. Therefore, this aspect should be taken into account during the design of the mold, to achieve easy and uniform temperature control [
30].
The experimental data regarding the defects analysis in the junction area of the semi-finished extruded billets, found the presence of two varieties of defects. The first featured aluminum flecks of debris adherent to the surface. The chemical composition confirmed that the origin of this debris was the extruded material, since it is identical. The adhesion between the dye and the extruded material generated these defects, as testified by their peculiar morphologies. These defects can thus be recognized as pick-ups [
23].
Further to this, a second category of defects was detected and it identified as dye pick-up. This observation was proven by the chemical composition analysis of the deposited material. Indeed, this composition is similar the composition of the AISI H13 tool steel, which is reported in
Table 7. Since the extrusion dye is made in this material, its provenance is clearly proved [
23,
24,
25,
26,
27].
Moreover, any metallurgical origin of the analyzed defects can be excluded. Indeed, both the SEM/BSE analysis and optical microscopy did not detect the presence of any other phases or intermetallic precipitates, any abnormal microstructural features or any abnormal chemical element concentrations. It follows that the origin of the detected defects is linked to wear issues and, in detail, it can be recognized as relating totally to the processing parameters and the tools [
23,
24,
25,
26,
27].
It should be highlighted that no correlation between the variation of extrusion parameters during the production trials and the species defects were identified. The generation of these kinds of defects would not be totally prevented using liquid nitrogen cooling of the extrusion dye. On the other hand, the occurrence of new defect classes can be excluded by using this technology.
In addition, the liquid nitrogen cooling of the extrusion dyes resulted in a beneficial effect on the amount and on the size of the extrusion defects with patent improvements. Indeed, although the analyzed extrusion defects were few and topologically isolated, the use of this technology further lowered their occurrence and dimensions [
11,
12].
However, considering their application, in which the aesthetic aspect plays a predominant role, even isolated and very small defects would cause the rejection of the extruded semi-product (
Figure 11). Taking into account the further steps involved in the realization of the finished product, we can set the defect acceptance threshold at a height of 15 μm. Indeed, since the paint layer does not follow the defect profile, it could cover and hide these small defects.
Considering this finishing process, the most significant roughness data were those describing the maxima of the roughness measurements of the surfaces and the distribution of the height histogram. These data (
Table 4,
Table 5 and
Table 6 and
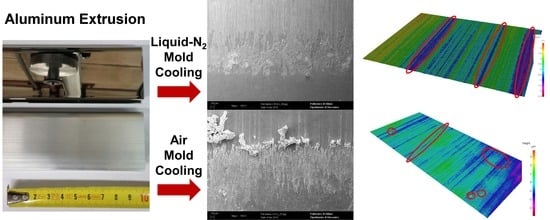
Figure 10) demonstrate the beneficial effect of the liquid nitrogen cooling of the extrusion dye, and an improvement in this key aspect can be appreciated. In addition, the surface roughness maps of the reconstructed surfaces likewise highlight this aspect (
Figure 12). The surface of the liquid nitrogen cooled specimen was very smooth and featured valleys along the extrusion direction. On the other hand, on the surface of the conventionally extruded semi-product, peak-like defects were detected emerging from the surface, harming the subsequent finishing processes.
Liquid nitrogen dye cooling technology improves the extrusion process through a complex mechanism, which can be described as follows: the debris, which generates the previously analyzed defects, is in both cases related to the adhesion phenomena occurring during extrusion between the dye and the flowing aluminum. However, this debris is much less present when the liquid nitrogen cools down the mold, even if the measured temperature is the same as for the air cooling. Hence, the improvement in the surface finishing, obtained through the liquid nitrogen cooling technology, is not only related to the lowering of the working temperature.
A different kind of contribution, which could increase the surface finishing, is given by the mechanical action that the nitrogen exerts on the flowing extruded material. Indeed, while absorbing the heat cooling the dye, nitrogen undergoes a phase transformation from the liquid to the gaseous state. This phase transition is known to be accompanied by a very large increase in volume. However, since the cooling circuit is pressured, the phase transformation generates a detaching pressure on the flowing material, which keeps it separated from the mold.
Moreover, liquid nitrogen cooling has another beneficial effect for surface finishing. In detail, it acts as an inert atmosphere enveloping the flowing material, avoiding its oxidation. This plays a central role in wear phenomena, in which aluminum is involved, since its oxide is very easily generated and is extremely hard. On the other hand, in comparison, aluminum is extremely soft and easily damageable with respect to its oxide [
4].
Therefore, these beneficial effects on surface finishing, related to the use of liquid nitrogen mold cooling, result from a combination of the cooling effect, the inertizing effect and a mechanical detaching pressure generated by the liquid-to-gaseous transition.