Chemoresistance to Valproate Treatment of Bovine Leukemia Virus-Infected Sheep; Identification of Improved HDAC Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reduction of B Cell Counts upon VPA Treatment

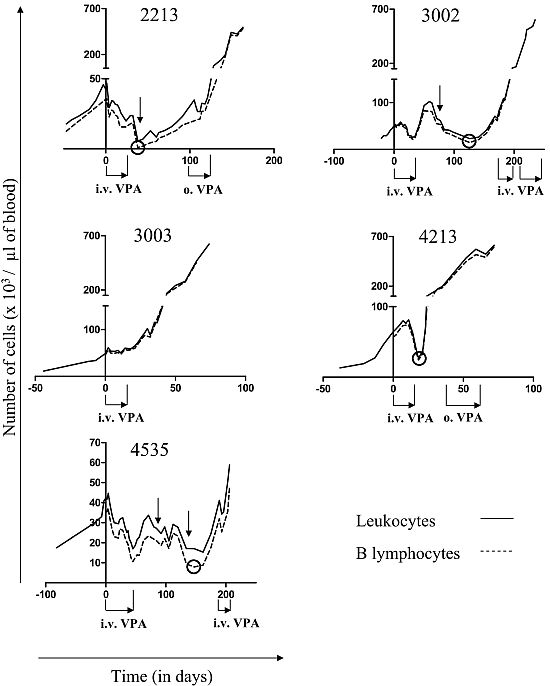
2.2. Relapse and Onset of Chemoresistance against VPA Treatment

2.3. Ex Vivo Cell Cycle Distribution and Viral Expression during VPA Treatment and Relapse
 ,
,  and
and  for respectively 1, 5 and 10mM of VPA) but clearly triggered apoptosis (Figure 3, right panels,
for respectively 1, 5 and 10mM of VPA) but clearly triggered apoptosis (Figure 3, right panels,  ,
,  and
and  for respectively 1, 5 and 10mM of VPA). Importantly, this proapoptotic effect persisted throughout VPA treatment as well as during relapse.
for respectively 1, 5 and 10mM of VPA). Importantly, this proapoptotic effect persisted throughout VPA treatment as well as during relapse. ,
,  and
and  correspond to 0, 1, 5 and 10 mM of VPA, respectively) and apoptosis (right panels; □,
correspond to 0, 1, 5 and 10 mM of VPA, respectively) and apoptosis (right panels; □,  ,
,  and
and  for 0, 1, 5 and 10 mM of VPA, respectively) were determined by flow cytometry. Absolute leukocyte numbers are indicated as reference (dotted line).
for 0, 1, 5 and 10 mM of VPA, respectively) were determined by flow cytometry. Absolute leukocyte numbers are indicated as reference (dotted line).
 ,
,  and
and  correspond to 0, 1, 5 and 10 mM of VPA, respectively) and apoptosis (right panels; □,
correspond to 0, 1, 5 and 10 mM of VPA, respectively) and apoptosis (right panels; □,  ,
,  and
and  for 0, 1, 5 and 10 mM of VPA, respectively) were determined by flow cytometry. Absolute leukocyte numbers are indicated as reference (dotted line).
for 0, 1, 5 and 10 mM of VPA, respectively) were determined by flow cytometry. Absolute leukocyte numbers are indicated as reference (dotted line).
 ,
,  , and
, and  for respectively 1, 5 and 10mM of VPA), most likely due to the proapototic effect of VPA. In contrast, the relative amount of p24 antigen increased most of the time (Figure 4, right panels,
for respectively 1, 5 and 10mM of VPA), most likely due to the proapototic effect of VPA. In contrast, the relative amount of p24 antigen increased most of the time (Figure 4, right panels,  ,
,  and
and  for respectively 1, 5 and 10mM of VPA) confirming previous observations [6]. Because apoptotic cells do not express viral proteins [2], the p24 burst is due to an increase of proviral expression.
for respectively 1, 5 and 10mM of VPA) confirming previous observations [6]. Because apoptotic cells do not express viral proteins [2], the p24 burst is due to an increase of proviral expression.2.4. Tumor Clone Replacement in Two out of Five BLV-Infected Sheep
2.5. Treatment of VPA-Unresponsive Animals with Other HDAC Inhibitors
 ,
,  , and
, and  for respectively 1, 5 and 10 mM of VPA) were determined by flow cytometry. The amount of p24 in the supernatant (right panels; ∇ for 0 mM,
for respectively 1, 5 and 10 mM of VPA) were determined by flow cytometry. The amount of p24 in the supernatant (right panels; ∇ for 0 mM,  ,
,  and
and  for respectively 1, 5 and 10 mM of VPA) was measured by ELISA. Data derives from optical densities measured in the linear range of a standard curve and are represented as relative amounts normalized for non-apoptotic B-cells. Absolute leukocyte numbers are indicated as reference (dotted line).
for respectively 1, 5 and 10 mM of VPA) was measured by ELISA. Data derives from optical densities measured in the linear range of a standard curve and are represented as relative amounts normalized for non-apoptotic B-cells. Absolute leukocyte numbers are indicated as reference (dotted line).
 ,
,  , and
, and  for respectively 1, 5 and 10 mM of VPA) were determined by flow cytometry. The amount of p24 in the supernatant (right panels; ∇ for 0 mM,
for respectively 1, 5 and 10 mM of VPA) were determined by flow cytometry. The amount of p24 in the supernatant (right panels; ∇ for 0 mM,  ,
,  and
and  for respectively 1, 5 and 10 mM of VPA) was measured by ELISA. Data derives from optical densities measured in the linear range of a standard curve and are represented as relative amounts normalized for non-apoptotic B-cells. Absolute leukocyte numbers are indicated as reference (dotted line).
for respectively 1, 5 and 10 mM of VPA) was measured by ELISA. Data derives from optical densities measured in the linear range of a standard curve and are represented as relative amounts normalized for non-apoptotic B-cells. Absolute leukocyte numbers are indicated as reference (dotted line).


2.6. Discussion
3. Experimental
3.1. Experimental Design of VPA Treatment
3.3. PCR Amplification of the BLV Provirus
3.4. Analysis of BLV Integration Sites by Southern Blot
3.5. Analysis of Apoptosis and Proliferation ex Vivo in Short Term Cultures of Peripheral Blood Mononuclear Cells
3.6. Analysis of Viral Expression ex Vivo by Flow Cytometry and ELISA
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Burny, A.; Bruck, C.; Cleuter, Y.; Couez, D.; Deschamps, J.; Gregoire, D.; Ghysdael, J.; Kettmann, R.; Mammerickx, M.; Marbaix, G. Bovine leukaemia virus and enzootic bovine leukosis. Onderstepoort. J. Vet. Res. 1985, 52, 133–144. [Google Scholar]
- Gillet, N.; Florins, A.; Boxus, M.; Burteau, C.; Nigro, A.; Vandermeers, F.; Balon, H.; Bouzar, A.B.; Defoiche, J.; Burny, A.; Reichert, M.; Kettmann, R.; Willems, L. Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human. Retrovirology 2007, 4, 18. [Google Scholar] [CrossRef]
- Kerkhofs, P.; Adam, E.; Droogmans, L.; Portetelle, D.; Mammerickx, M.; Burny, A.; Kettmann, R.; Willems, L. Cellular pathways involved in the ex vivo expression of bovine leukemia virus. J. Virol. 1996, 70, 2170–2177. [Google Scholar]
- Merezak, C.; Reichert, M.; Van Lint, C.; Kerkhofs, P.; Portetelle, D.; Willems, L.; Kettmann, R. Inhibition of histone deacetylases induces bovine leukemia virus expression in vitro and in vivo. J. Virol. 2002, 76, 5034–5042. [Google Scholar] [CrossRef]
- Florins, A.; Gillet, N.; Asquith, B.; Boxus, M.; Burteau, C.; Twizere, J.C.; Urbain, P.; Vandermeers, F.; Debacq, C.; Sanchez-Alcaraz, M.T.; Schwartz-Cornil, I.; Kerkhofs, P.; Jean, G.; Thewis, A.; Hay, J.; Mortreux, F.; Wattel, E.; Reichert, M.; Burny, A.; Kettmann, R.; Bangham, C.; Willems, L. Cell dynamics and immune response to BLV infection: a unifying model. Front Biosci. 2007, 12, 1520–1531. [Google Scholar] [CrossRef]
- Achachi, A.; Florins, A.; Gillet, N.; Debacq, C.; Urbain, P.; Foutsop, G.M.; Vandermeers, F.; Jasik, A.; Reichert, M.; Kerkhofs, P.; Lagneaux, L.; Burny, A.; Kettmann, R.; Willems, L. Valproate activates bovine leukemia virus gene expression, triggers apoptosis, and induces leukemia/lymphoma regression in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 10309–10314. [Google Scholar]
- Lezin, A.; Gillet, N.; Olindo, S.; Signate, A.; Grandvaux, N.; Verlaeten, O.; Belrose, G.; de Carvalho Bittencourt, M.; Hiscott, J.; Asquith, B.; Burny, A.; Smadja, D.; Cesaire, R.; Willems, L. Histone deacetylase mediated transcriptional activation reduces proviral loads in HTLV-1 associated myelopathy/tropical spastic paraparesis patients. Blood 2007, 110, 3722–3728. [Google Scholar]
- Olindo, S.; Belrose, G.; Gillet, N.; Rodriguez, S.; Boxus, M.; Verlaeten, O.; Asquith, B.; Bangham, C.; Signate, A.; Smadja, D.; Lezin, A.; Cesaire, R.; Willems, L. Safety of long-term treatment of HAM/TSP patients with valproic acid. Blood 2011, 118, 6306–6309. [Google Scholar]
- Proietti, F.A.; Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Murphy, E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005, 24, 6058–6068. [Google Scholar] [CrossRef]
- Zimmerman, B.; Sargeant, A.; Landes, K.; Fernandez, S.A.; Chen, C.S.; Lairmore, M.D. Efficacy of novel histone deacetylase inhibitor, AR42, in a mouse model of, human T-lymphotropic virus type 1 adult T cell lymphoma. Leukemia Res. 2011, 35, 1491–1497. [Google Scholar] [CrossRef]
- Hasegawa, H.; Yamada, Y.; Tsukasaki, K.; Mori, N.; Tsuruda, K.; Sasaki, D.; Usui, T.; Osaka, A.; Atogami, S.; Ishikawa, C.; Machijima, Y.; Sawada, S.; Hayashi, T.; Miyazaki, Y.; Kamihira, S. LBH589, a deacetylase inhibitor, induces apoptosis in adult T-cell leukemia/lymphoma cells via activation of a novel RAIDD-caspase-2 pathway. Leukemia 2011, 25, 575–587. [Google Scholar] [CrossRef]
- Nishioka, C.; Ikezoe, T.; Yang, J.; Komatsu, N.; Bandobashi, K.; Taniguchi, A.; Kuwayama, Y.; Togitani, K.; Koeffler, H.P.; Taguchi, H. Histone deacetylase inhibitors induce growth arrest and apoptosis of HTLV-1-infected T-cells via blockade of signaling by nuclear factor kappaB. Leukemia Res. 2008, 32, 287–296. [Google Scholar]
- Ramos, J.T.; Diaz, L.; Ruiz, P.; Barber, G.; Harrington, W. Targeting HTLV-I latency in Adult T-cell Leukemia/Lymphoma. Retrovirology 2011, 8 (Suppl. 1), A48. [Google Scholar]
- Florins, A.; de Brogniez, A.; Elemans, M.; Bouzar, A.B.; Francois, C.; Reichert, M.; Asquith, B.; Willems, L. Viral expression directs the fate of B cells in bovine leukemia virus-infected sheep. J. Virol. 2012, 86, 621–624. [Google Scholar] [CrossRef]
- Merimi, M.; Klener, P.; Szynal, M.; Cleuter, Y.; Kerkhofs, P.; Burny, A.; Martiat, P.; Van den Broeke, A. Suppression of viral gene expression in bovine leukemia virus-associated B-cell malignancy: interplay of epigenetic modifications leading to chromatin with a repressive histone code. J. Virol. 2007, 81, 5929–5939. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Nau, H.; Michaelis, M.; Cinatl, J., Jr. Valproate and valproate-analogues: potent tools to fight against cancer. Curr. Med. Chem. 2002, 9, 1417–1433. [Google Scholar]
- Kostrouchova, M.; Kostrouch, Z. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol. (Praha) 2007, 53, 37–49. [Google Scholar]
- Insinga, A.; Monestiroli, S.; Ronzoni, S.; Gelmetti, V.; Marchesi, F.; Viale, A.; Altucci, L.; Nervi, C.; Minucci, S.; Pelicci, P.G. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat. Med. 2005, 11, 71–76. [Google Scholar] [CrossRef]
- Nebbioso, A.; Clarke, N.; Voltz, E.; Germain, E.; Ambrosino, C.; Bontempo, P.; Alvarez, R.; Schiavone, E.M.; Ferrara, F.; Bresciani, F.; Weisz, A.; de Lera, A.R.; Gronemeyer, H.; Altucci, L. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 2005, 11, 77–84. [Google Scholar] [CrossRef]
- Vandermeers, F.; Hubert, P.; Delvenne, P.; Mascaux, C.; Grigoriu, B.; Burny, A.; Scherpereel, A.; Willems, L. Valproate, in combination with pemetrexed and cisplatin, provides additional efficacy to the treatment of malignant mesothelioma. Clin.Cancer Res. 2009, 15, 2818–2828. [Google Scholar] [CrossRef]
- Debacq, C.; Gillet, N.; Asquith, B.; Sanchez-Alcaraz, M.T.; Florins, A.; Boxus, M.; Schwartz-Cornil, I.; Bonneau, M.; Jean, G.; Kerkhofs, P.; Hay, J.; Thewis, A.; Kettmann, R.; Willems, L. Peripheral blood B-cell death compensates for excessive proliferation in lymphoid tissues and maintains homeostasis in bovine leukemia virus-infected sheep. J. Virol. 2006, 80, 9710–9719. [Google Scholar]
- Florins, A.; Gillet, N.; Asquith, B.; Debacq, C.; Jean, G.; Schwartz-Cornil, I.; Bonneau, M.; Burny, A.; Reichert, M.; Kettmann, R.; Willems, L. Spleen-dependent turnover of CD11b peripheral blood B lymphocytes in bovine leukemia virus-infected sheep. J. Virol. 2006, 80, 11998–12008. [Google Scholar]
- Granneman, G.R.; Wang, S.I.; Machinist, J.M.; Kesterson, J.W. Aspects of the metabolism of valproic acid. Xenobiotica 1984, 14, 375–387. [Google Scholar] [CrossRef]
- Wong, H.; Rurak, D.W.; Kumar, S.; Kwan, E.; Abbott, F.S.; Riggs, K.W. Dose-dependent pharmacokinetics and metabolism of valproic acid in newborn lambs and adult sheep. Drug Metab. Dispos. 2001, 29, 664–675. [Google Scholar]
- Wong, H.; Kumar, S.; Rurak, D.W.; Kwan, E.; Abbott, F.S.; Riggs, K.W. Ontogeny of valproic acid disposition and metabolism: a developmental study in postnatal lambs and adult sheep. Drug Metab. Dispos. 2000, 28, 912–919. [Google Scholar]
- Dequiedt, F.; Kettmann, R.; Burny, A.; Willems, L. Mutations in the p53 tumor-suppressor gene are frequently associated with bovine leukemia virus-induced leukemogenesis in cattle but not in sheep. Virology 1995, 209, 676–683. [Google Scholar] [CrossRef]
- Gatti, L.; Zunino, F. Overview of tumor cell chemoresistance mechanisms. Methods Mol. Med. 2005, 111, 127–148. [Google Scholar]
- Huang, Y.; Sadee, W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006, 239, 168–182. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Adeyeye, M.C.; Yang, Z.; Shen, D.D. Valproic acid uptake by bovine brain microvessel endothelial cells: role of active efflux transport. Epilepsy Res. 2004, 58, 53–66. [Google Scholar] [CrossRef]
- Tang, R.; Faussat, A.M.; Majdak, P.; Perrot, J.Y.; Chaoui, D.; Legrand, O.; Marie, J.P. Valproic acid inhibits proliferation and induces apoptosis in acute myeloid leukemia cells expressing P-gp and MRP1. Leukemia 2004, 18, 1246–1251. [Google Scholar] [CrossRef]
- Baltes, S.; Fedrowitz, M.; Tortos, C.L.; Potschka, H.; Loscher, W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J. Pharmacol. Exp. Ther. 2007, 320, 331–343. [Google Scholar]
- Afonso, P.V.; Mekaouche, M.; Mortreux, F.; Toulza, F.; Moriceau, A.; Wattel, E.; Gessain, A.; Bangham, C.R.; Dubreuil, G.; Plumelle, Y.; Hermine, O.; Estaquier, J.; Mahieux, R. Highly active antiretroviral treatment against STLV-1 infection combining reverse transcriptase and HDAC inhibitors. Blood 2010, 116, 3802–3808. [Google Scholar] [CrossRef]
- Uozumi, K. Treatment of adult T-cell leukemia. J. Clin.Exp. Hemat. : JCEH 2010, 50, 9–25. [Google Scholar] [CrossRef]
- Hishizawa, M.; Kanda, J.; Utsunomiya, A.; Taniguchi, S.; Eto, T.; Moriuchi, Y.; Tanosaki, R.; Kawano, F.; Miyazaki, Y.; Masuda, M.; Nagafuji, K.; Hara, M.; Takanashi, M.; Kai, S.; Atsuta, Y.; Suzuki, R.; Kawase, T.; Matsuo, K.; Nagamura-Inoue, T.; Kato, S.; Sakamaki, H.; Morishima, Y.; Okamura, J.; Ichinohe, T.; Uchiyama, T. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood 2010, 116, 1369–1376. [Google Scholar]
- Bazarbachi, A.; Plumelle, Y.; Carlos Ramos, J.; Tortevoye, P.; Otrock, Z.; Taylor, G.; Gessain, A.; Harrington, W.; Panelatti, G.; Hermine, O. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 2010, 28, 4177–4183. [Google Scholar]
- Nasr, R.; El Hajj, H.; Kfoury, Y.; de The, H.; Hermine, O.; Bazarbachi, A. Controversies in targeted therapy of adult T cell leukemia/lymphoma: ON target or OFF target effects? Viruses 2011, 3, 750–769. [Google Scholar] [CrossRef]
- Willems, L.; Kettmann, R.; Dequiedt, F.; Portetelle, D.; Voneche, V.; Cornil, I.; Kerkhofs, P.; Burny, A.; Mammerickx, M. In vivo infection of sheep by bovine leukemia virus mutants. J. Virol. 1993, 67, 4078–4085. [Google Scholar]
- Lew, A.E.; Bock, R.E.; Miles, J.; Cuttell, L.B.; Steer, P.; Nadin-Davis, S.A. Sensitive and specific detection of bovine immunodeficiency virus and bovine syncytial virus by 5' Taq nuclease assays with fluorescent 3' minor groove binder-DNA probes. J. Virol. Methods 2004, 116, 1–9. [Google Scholar] [CrossRef]
- Debacq, C.; Sanchez Alcaraz, M.T.; Mortreux, F.; Kerkhofs, P.; Kettmann, R.; Willems, L. Reduced proviral loads during primo-infection of sheep by Bovine Leukemia virus attenuated mutants. Retrovirology 2004, 1, 31. [Google Scholar] [CrossRef] [Green Version]
Supplementary Material

© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gillet, N.; Vandermeers, F.; De Brogniez, A.; Florins, A.; Nigro, A.; François, C.; Bouzar, A.-B.; Verlaeten, O.; Stern, E.; Lambert, D.M.; et al. Chemoresistance to Valproate Treatment of Bovine Leukemia Virus-Infected Sheep; Identification of Improved HDAC Inhibitors. Pathogens 2012, 1, 65-82. https://doi.org/10.3390/pathogens1020065
Gillet N, Vandermeers F, De Brogniez A, Florins A, Nigro A, François C, Bouzar A-B, Verlaeten O, Stern E, Lambert DM, et al. Chemoresistance to Valproate Treatment of Bovine Leukemia Virus-Infected Sheep; Identification of Improved HDAC Inhibitors. Pathogens. 2012; 1(2):65-82. https://doi.org/10.3390/pathogens1020065
Chicago/Turabian StyleGillet, Nicolas, Fabian Vandermeers, Alix De Brogniez, Arnaud Florins, Annamaria Nigro, Carole François, Amel-Baya Bouzar, Olivier Verlaeten, Eric Stern, Didier M. Lambert, and et al. 2012. "Chemoresistance to Valproate Treatment of Bovine Leukemia Virus-Infected Sheep; Identification of Improved HDAC Inhibitors" Pathogens 1, no. 2: 65-82. https://doi.org/10.3390/pathogens1020065
APA StyleGillet, N., Vandermeers, F., De Brogniez, A., Florins, A., Nigro, A., François, C., Bouzar, A. -B., Verlaeten, O., Stern, E., Lambert, D. M., Wouters, J., & Willems, L. (2012). Chemoresistance to Valproate Treatment of Bovine Leukemia Virus-Infected Sheep; Identification of Improved HDAC Inhibitors. Pathogens, 1(2), 65-82. https://doi.org/10.3390/pathogens1020065