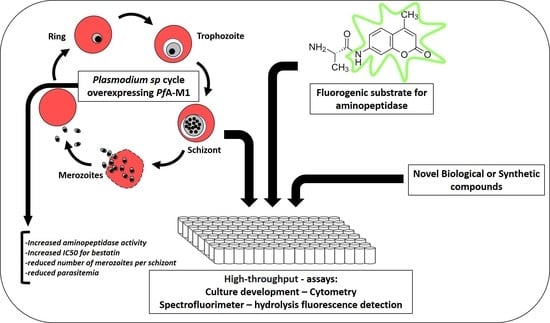
Overexpression of Plasmodium falciparum M1 Aminopeptidase Promotes an Increase in Intracellular Proteolysis and Modifies the Asexual Erythrocytic Cycle Development
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. P. falciparum Culture and Synchronization
4.2. Cloning of PfA-M1 Gene for Overexpression in P. falciparum
4.3. Transfection and Selection of PfA-M1-Overexpressing P. falciparum
4.4. Western Blot and Microscopic Detection of overPfA-M1 Parasites
4.5. Assessment of overPfA-M1 Aminopeptidase Activity, Inhibition by Bestatin and Relationship with Ca2+ Mobilizers and E64d in Isolated Live Parasites
4.6. In Vitro Antimalarial Activity Assays
4.7. Morphology Analysis of P. falciparum
4.8. Analysis of Parasitemia in P. falciparum
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2020; p. 185. Available online: https://www.who.int/publications/i/item/9789241565721 (accessed on 5 November 2021).
- Kantele, A.; Jokiranta, T.S. Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin. Infect. Dis. 2011, 52, 1356–1362. [Google Scholar] [CrossRef]
- Miller, L.H.; Ackerman, H.C.; Su, X.Z.; Wellems, T.E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 2013, 19, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Phyo, A.P.; Nkhoma, S.; Stepniewska, K.; Ashley, E.A.; Nair, S.; McGready, R.; ler Moo, C.; Al-Saai, S.; Dondorp, A.M.; Lwin, K.M.; et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: A longitudinal study. Lancet 2012, 379, 1960–1966. [Google Scholar] [CrossRef] [Green Version]
- Amino, R.; Thiberge, S.; Martin, B.; Celli, S.; Shorte, S.; Frischknecht, F.; Menard, R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 2006, 12, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.J. Proteases of protozoan parasites. Adv. Parasitol. 1999, 43, 105–159. [Google Scholar] [CrossRef]
- Deu, E. Proteases as antimalarial targets: Strategies for genetic, chemical, and therapeutic validation. FEBS J. 2017, 284, 2604–2628. [Google Scholar] [CrossRef]
- Flipo, M.; Beghyn, T.; Leroux, V.; Florent, I.; Deprez, B.P.; Deprez-Poulain, R.F. Novel selective inhibitors of the zinc plasmodial aminopeptidase PfA-M1 as potential antimalarial agents. J. Med. Chem. 2007, 50, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Kolakovich, K.A.; Gluzman, I.Y.; Duffin, K.L.; Goldberg, D.E. Generation of hemoglobin peptides in the acidic digestive vacuole of Plasmodium falciparum implicates peptide transport in amino acid production. Mol. Biochem. Parasitol. 1997, 87, 123–135. [Google Scholar] [CrossRef]
- Gavigan, C.S.; Dalton, J.P.; Bell, A. The role of aminopeptidases in haemoglobin degradation in Plasmodium falciparum-infected erythrocytes. Mol. Biochem. Parasitol. 2001, 117, 37–48. [Google Scholar] [CrossRef]
- Dalal, S.; Klemba, M. Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J. Biol. Chem. 2007, 282, 35978–35987. [Google Scholar] [CrossRef] [Green Version]
- Janka, L.; Clemente, J.; Vaiana, N.; Sparatore, A.; Romeo, S.; Dunn, B.M. Targeting the plasmepsin 4 orthologs of Plasmodium sp. with “double drug” inhibitors. Protein Pept. Lett. 2008, 15, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, J.; Rohrbach, P.; Dalton, J.P. The malaria digestive vacuole. Front. Biosci. 2012, 4, 1424–1448. [Google Scholar] [CrossRef] [Green Version]
- Biagini, G.A.; Bray, P.G.; Spiller, D.G.; White, M.R.; Ward, S.A. The digestive food vacuole of the malaria parasite is a dynamic intracellular Ca2+ store. J. Biol. Chem. 2003, 278, 27910–27915. [Google Scholar] [CrossRef] [Green Version]
- Moreno, S.N.; Ayong, L.; Pace, D.A. Calcium storage and function in apicomplexan parasites. Essays Biochem. 2011, 51, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Lourido, S.; Moreno, S.N. The calcium signaling toolkit of the Apicomplexan parasites Toxoplasma gondii and Plasmodium spp. Cell Calcium. 2015, 57, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Gazarini, M.L.; Garcia, C.R. The malaria parasite mitochondrion senses cytosolic Ca2+ fluctuations. Biochem. Biophys. Res. Commun. 2004, 321, 138–144. [Google Scholar] [CrossRef]
- Gazarini, M.L.; Thomas, A.P.; Pozzan, T.; Garcia, C.R. Calcium signaling in a low calcium environment: How the intracellular malaria parasite solves the problem. J. Cell Biol. 2003, 161, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Varotti, F.P.; Beraldo, F.H.; Gazarini, M.L.; Garcia, C.R. Plasmodium falciparum malaria parasites display a THG-sensitive Ca2+ pool. Cell Calcium. 2003, 33, 137–144. [Google Scholar] [CrossRef]
- Farias, S.L.; Gazarini, M.L.; Melo, R.L.; Hirata, I.Y.; Juliano, M.A.; Juliano, L.; Garcia, C.R. Cysteine-protease activity elicited by Ca2+ stimulus in Plasmodium. Mol. Biochem. Parasitol. 2005, 141, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.M.; Budu, A.; Ventura, P.D.; Bagnaresi, P.; Cotrin, S.S.; Cunha, R.L.; Carmona, A.K.; Juliano, L.; Gazarini, M.L. Specific calpain activity evaluation in Plasmodium parasites. Anal. Biochem. 2015, 468, 22–27. [Google Scholar] [CrossRef]
- Gomes Smaul, M.M.; Budu, A.; Montagna, G.N.; Fernanda da Silva Ferrara, T.; El Chamy Maluf, S.; Bagnaresi, P.; Ferreira Machado, M.M.; Bronze Dos Santos, F.; Ferreira de Azevedo, M.; Carmona, A.K.; et al. Plasmodium falciparum histidine triad protein and calmodulin modulates calcium homeostasis and intracellular proteolysis. Biochem. Biophys. Res. Commun. 2018, 503, 722–728. [Google Scholar] [CrossRef]
- Budu, A.; Gomes, M.M.; Melo, P.M.; El Chamy Maluf, S.; Bagnaresi, P.; Azevedo, M.F.; Carmona, A.K.; Gazarini, M.L. Calmidazolium evokes high calcium fluctuations in Plasmodium falciparum. Cell Signal. 2016, 28, 125–135. [Google Scholar] [CrossRef]
- Vaughan, M.D.; Sampson, P.B.; Honek, J.F. Methionine in and out of proteins: Targets for drug design. Curr. Med. Chem. 2002, 9, 385–409. [Google Scholar] [CrossRef]
- Cunningham, E.; Drag, M.; Kafarski, P.; Bell, A. Chemical target validation studies of aminopeptidase in malaria parasites using alpha-aminoalkylphosphonate and phosphonopeptide inhibitors. Antimicrob. Agents Chemother. 2008, 52, 3221–3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bounaadja, L.; Schmitt, M.; Albrecht, S.; Mouray, E.; Tarnus, C.; Florent, I. Selective inhibition of PfA-M1, over PfA-M17, by an amino-benzosuberone derivative blocks malaria parasites development in vitro and in vivo. Malar. J. 2017, 16, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Bacerio, J.; Maluf, S.E.C.; Mendez, Y.; Pascual, I.; Florent, I.; Melo, P.M.S.; Budu, A.; Ferreira, J.C.; Moreno, E.; Carmona, A.K.; et al. KBE009: An antimalarial bestatin-like inhibitor of the Plasmodium falciparum M1 aminopeptidase discovered in an Ugi multicomponent reaction-derived peptidomimetic library. Bioorg. Med. Chem. 2017, 25, 4628–4636. [Google Scholar] [CrossRef]
- Drinkwater, N.; Lee, J.; Yang, W.; Malcolm, T.R.; McGowan, S. M1 aminopeptidases as drug targets: Broad applications or therapeutic niche? FEBS J. 2017, 284, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, O.; Sow, C.; Geze, M.; Nyalwidhe, J.; Florent, I. Plasmodium falciparum PfA-M1 aminopeptidase is trafficked via the parasitophorous vacuole and marginally delivered to the food vacuole. Malar. J. 2010, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Ragheb, D.; Dalal, S.; Bompiani, K.M.; Ray, W.K.; Klemba, M. Distribution and biochemical properties of an M1-family aminopeptidase in Plasmodium falciparum indicate a role in vacuolar hemoglobin catabolism. J. Biol. Chem. 2011, 286, 27255–27265. [Google Scholar] [CrossRef] [Green Version]
- Mathew, R.; Wunderlich, J.; Thivierge, K.; Cwiklinski, K.; Dumont, C.; Tilley, L.; Rohrbach, P.; Dalton, J.P. Biochemical and cellular characterisation of the Plasmodium falciparum M1 alanyl aminopeptidase (PfM1AAP) and M17 leucyl aminopeptidase (PfM17LAP). Sci. Rep. 2021, 11, 2854. [Google Scholar] [CrossRef]
- Poreba, M.; McGowan, S.; Skinner-Adams, T.S.; Trenholme, K.R.; Gardiner, D.L.; Whisstock, J.C.; To, J.; Salvesen, G.S.; Dalton, J.P.; Drag, M. Fingerprinting the substrate specificity of M1 and M17 aminopeptidases of human malaria, Plasmodium falciparum. PLoS ONE 2012, 7, e31938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanenko, A.A.; Heng, H.H. Transient and stable vector transfection: Pitfalls, off-target effects, artifacts. Mutat. Res. Rev. Mutat. Res. 2017, 773, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Harbut, M.B.; Velmourougane, G.; Dalal, S.; Reiss, G.; Whisstock, J.C.; Onder, O.; Brisson, D.; McGowan, S.; Klemba, M.; Greenbaum, D.C. Bestatin-based chemical biology strategy reveals distinct roles for malaria M1- and M17-family aminopeptidases. Proc. Natl. Acad Sci. USA 2011, 108, E526–E534. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 2018, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, S.; Porter, C.J.; Lowther, J.; Stack, C.M.; Golding, S.J.; Skinner-Adams, T.S.; Trenholme, K.R.; Teuscher, F.; Donnelly, S.M.; Grembecka, J.; et al. Structural basis for the inhibition of the essential Plasmodium falciparum M1 neutral aminopeptidase. Proc. Natl. Acad. Sci. USA 2009, 106, 2537–2542. [Google Scholar] [CrossRef] [Green Version]
- Ringwald, P.; Meche, F.S.; Bickii, J.; Basco, L.K. In vitro culture and drug sensitivity assay of Plasmodium falciparum with nonserum substitute and acute-phase sera. J. Clin. Microbiol. 1999, 37, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, D.L.; Trenholme, K.R.; Skinner-Adams, T.S.; Stack, C.M.; Dalton, J.P. Overexpression of leucyl aminopeptidase in Plasmodium falciparum parasites. Target for the antimalarial activity of bestatin. J. Biol. Chem. 2006, 281, 1741–1745. [Google Scholar] [CrossRef] [Green Version]
- Skinner-Adams, T.S.; Lowther, J.; Teuscher, F.; Stack, C.M.; Grembecka, J.; Mucha, A.; Kafarski, P.; Trenholme, K.R.; Dalton, J.P.; Gardiner, D.L. Identification of phosphinate dipeptide analog inhibitors directed against the Plasmodium falciparum M17 leucine aminopeptidase as lead antimalarial compounds. J. Med. Chem. 2007, 50, 6024–6031. [Google Scholar] [CrossRef]
- Humberstone, A.J.; Cowman, A.F.; Horton, J.; Charman, W.N. Effect of altered serum lipid concentrations on the IC50 of halofantrine against Plasmodium falciparum. J. Pharm. Sci. 1998, 87, 256–258. [Google Scholar] [CrossRef]
- Allary, M.; Schrevel, J.; Florent, I. Properties, stage-dependent expression and localization of Plasmodium falciparum M1 family zinc-aminopeptidase. Parasitology 2002, 125, 1–10. [Google Scholar] [CrossRef]
- Lew, V.L.; Macdonald, L.; Ginsburg, H.; Krugliak, M.; Tiffert, T. Excess haemoglobin digestion by malaria parasites: A strategy to prevent premature host cell lysis. Blood Cells Mol. Dis. 2004, 32, 353–359. [Google Scholar] [CrossRef]
- Constam, D.B.; Tobler, A.R.; Rensing-Ehl, A.; Kemler, I.; Hersh, L.B.; Fontana, A. Puromycin-sensitive aminopeptidase. Sequence analysis, expression, and functional characterization. J. Biol. Chem. 1995, 270, 26931–26939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Bacerio, J.; Osuna, J.; Ponce, A.; Fando, R.; Figarella, K.; Mendez, Y.; Charli, J.L.; Chavez Mde, L. High-level expression in Escherichia coli, purification and kinetic characterization of Plasmodium falciparum M1-aminopeptidase. Protein Expr. Purif. 2014, 104, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yayon, A.; Cabantchik, Z.I.; Ginsburg, H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984, 3, 2695–2700. [Google Scholar] [CrossRef]
- Lee, A.H.; Dhingra, S.K.; Lewis, I.A.; Singh, M.K.; Siriwardana, A.; Dalal, S.; Rubiano, K.; Klein, M.S.; Baska, K.S.; Krishna, S.; et al. Evidence for Regulation of Hemoglobin Metabolism and Intracellular Ionic Flux by the Plasmodium falciparum Chloroquine Resistance Transporter. Sci. Rep. 2018, 8, 13578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alleva, L.M.; Kirk, K. Calcium regulation in the intraerythrocytic malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 2001, 117, 121–128. [Google Scholar] [CrossRef]
- Glushakova, S.; Mazar, J.; Hohmann-Marriott, M.F.; Hama, E.; Zimmerberg, J. Irreversible effect of cysteine protease inhibitors on the release of malaria parasites from infected erythrocytes. Cell Microbiol. 2009, 11, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Azevedo, M.F.; Nie, C.Q.; Elsworth, B.; Charnaud, S.C.; Sanders, P.R.; Crabb, B.S.; Gilson, P.R. Plasmodium falciparum transfected with ultra bright NanoLuc luciferase offers high sensitivity detection for the screening of growth and cellular trafficking inhibitors. PLoS ONE 2014, 9, e112571. [Google Scholar] [CrossRef]
- Fidock, D.A.; Wellems, T.E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 1997, 94, 10931–10936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Byzia, A.; Szeffler, A.; Kalinowski, L.; Drag, M. Activity profiling of aminopeptidases in cell lysates using a fluorogenic substrate library. Biochimie 2016, 122, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Schuck, D.C.; Ribeiro, R.Y.; Nery, A.A.; Ulrich, H.; Garcia, C.R. Flow cytometry as a tool for analyzing changes in Plasmodium falciparum cell cycle following treatment with indol compounds. Cytom. A 2011, 79, 959–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoff, C.C.; Azevedo, M.F.; Thurler, A.B.; Maluf, S.E.C.; Melo, P.M.S.; del Rivero, M.A.; González-Bacerio, J.; Carmona, A.K.; Budu, A.; Gazarini, M.L. Overexpression of Plasmodium falciparum M1 Aminopeptidase Promotes an Increase in Intracellular Proteolysis and Modifies the Asexual Erythrocytic Cycle Development. Pathogens 2021, 10, 1452. https://doi.org/10.3390/pathogens10111452
Hoff CC, Azevedo MF, Thurler AB, Maluf SEC, Melo PMS, del Rivero MA, González-Bacerio J, Carmona AK, Budu A, Gazarini ML. Overexpression of Plasmodium falciparum M1 Aminopeptidase Promotes an Increase in Intracellular Proteolysis and Modifies the Asexual Erythrocytic Cycle Development. Pathogens. 2021; 10(11):1452. https://doi.org/10.3390/pathogens10111452
Chicago/Turabian StyleHoff, Carolina C., Mauro F. Azevedo, Adriana B. Thurler, Sarah El Chamy Maluf, Pollyana M. S. Melo, Maday Alonso del Rivero, Jorge González-Bacerio, Adriana K. Carmona, Alexandre Budu, and Marcos L. Gazarini. 2021. "Overexpression of Plasmodium falciparum M1 Aminopeptidase Promotes an Increase in Intracellular Proteolysis and Modifies the Asexual Erythrocytic Cycle Development" Pathogens 10, no. 11: 1452. https://doi.org/10.3390/pathogens10111452
APA StyleHoff, C. C., Azevedo, M. F., Thurler, A. B., Maluf, S. E. C., Melo, P. M. S., del Rivero, M. A., González-Bacerio, J., Carmona, A. K., Budu, A., & Gazarini, M. L. (2021). Overexpression of Plasmodium falciparum M1 Aminopeptidase Promotes an Increase in Intracellular Proteolysis and Modifies the Asexual Erythrocytic Cycle Development. Pathogens, 10(11), 1452. https://doi.org/10.3390/pathogens10111452