Toxocara canis and Toxocara cati Somatic and Excretory-Secretory Antigens Are Recognised by C-Type Lectin Receptors
Abstract
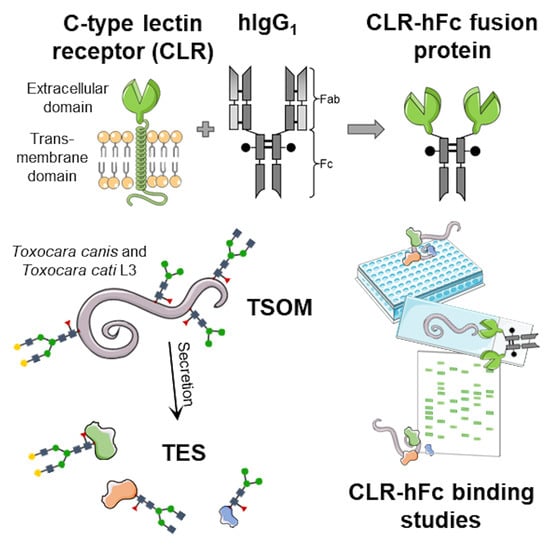
:1. Introduction
2. Results
2.1. CLR Binding to Toxocara spp. TSOM and TES
2.2. MGL-1 but Not MCL Binds to Toxocara spp. Larvae
2.3. Interaction of MGL-1 and MCL with Toxocara TSOM and TES in CLR-hFc Immunoblot
2.4. MGL-1 Does Not Influence Toxocara spp.-Mediated Dendritic Cell Effector Functions
2.5. Relevance of MCL on Toxocara spp.-Mediated Dendritic Cell Effector Functions
3. Discussion
4. Materials and Methods
4.1. Preparation of Toxocara spp. Antigens
4.2. CLR-hFc Fusion Proteins for Detection of CLR/Toxocara spp. Interactions
4.3. CLR-hFc ELISA Binding Assays
4.4. Fluorescence Microscopy-Based Binding Assay to Toxocara Larvae
4.5. CLR-hFc Recognition of Toxocara Antigen Fractions
4.6. Preparation of Bone Marrow Cells
4.7. MGL-1−/− and MCL−/− BMDC Stimulation Assays
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macpherson, C.N. The epidemiology and public health importance of toxocariasis: A zoonosis of global importance. Int. J. Parasitol. 2013, 43, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Bowman, D.D. Visceral larval migrans of Toxocara canis and Toxocara cati in non-canid and non-felid hosts. Adv. Parasitol. 2020, 109, 63–88. [Google Scholar] [CrossRef] [PubMed]
- CDC. Parasites—Neglected Parasitic Infections (NPIs). Available online: https://www.cdc.gov/parasites/npi/index.html (accessed on 31 January 2021).
- Woodhall, D.M.; Eberhard, M.L.; Parise, M.E. Neglected Parasitic Infections in the United States: Toxocariasis. Am. J. Trop. Med. Hyg. 2014, 90, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Auer, H.; Walochnik, J. Toxocariasis and the clinical spectrum. Adv. Parasitol. 2020, 109, 111–130. [Google Scholar] [CrossRef]
- Maizels, R.M. Toxocara canis: Molecular basis of immune recognition and evasion. Veter. Parasitol. 2013, 193, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaver, P.C.; Snyder, C.H.; Carrera, G.M.; Dent, J.H.; Lafferty, J.W. Chronic eosinophilia due to visceral larva migrans: Report of three cases. Pediatrics 1952, 9, 7–19. [Google Scholar] [PubMed]
- Mazur-Melewska, K.; Jończyk, K.; Modlińska-Cwalińska, A.; Figlerowicz, M.; Służewski, W. Visceral larva migrans syndrome: Analysis of serum cytokine levels in children with hepatic lesions confirmed in radiological findings. Parasite Immunol. 2014, 36, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Melewska, K.; Mania, A.; Sluzewski, W.; Figlerowicz, M. Clinical pathology of larval toxocariasis. Adv. Parasitol. 2020, 109, 153–163. [Google Scholar] [CrossRef]
- Nagy, D.; Bede, O.; Danka, J.; Szénási, Z.; Sipka, S. Analysis of serum cytokine levels in children with chronic cough associated with Toxocara canis infection. Parasite Immunol. 2012, 34, 581–588. [Google Scholar] [CrossRef]
- Resende, N.M.; Gazzinelli-Guimarães, P.H.; Barbosa, F.S.; Oliveira, L.M.; Nogueira, D.S.; Gazzinelli-Guimarães, A.C.; Gon-çalves, M.T.; Amorim, C.C.; Oliveira, F.M.; Caliari, M.V.; et al. New insights into the immunopathology of early Toxocara canis infection in mice. Parasit Vectors 2015, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Waindok, P.; Strube, C. Neuroinvasion of Toxocara canis- and T. cati-larvae mediates dynamic changes in brain cytokine and chemokine profile. J. Neuroinflamm. 2019, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zibaei, M.; Shayesteh, Z.; Moradi, N.; Bahadory, S. Human Toxocara Infection: Allergy and Immune Responses. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 18, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- Lightowlers, M.W.; Rickard, M.D. Excretory–secretory products of helminth parasites: Effects on host immune responses. Parasitology 1988, 96, S123–S166. [Google Scholar] [CrossRef]
- Mayer, S.; Raulf, M.-K.; Lepenies, B. C-type lectins: Their network and roles in pathogen recognition and immunity. Histochem. Cell Biol. 2017, 147, 223–237. [Google Scholar] [CrossRef]
- Gems, D.; Ferguson, C.J.; Robertson, B.D.; Nieves, R.; Page, A.P.; Blaxter, M.L.; Maizels, R.M. An Abundant, trans-spliced mRNA from Toxocara canis Infective Larvae Encodes a 26-kDa Protein with Homology to Phosphatidylethanolamine-binding Proteins. J. Biol. Chem. 1995, 270, 18517–18522. [Google Scholar] [CrossRef] [Green Version]
- Gems, D.; Maizels, R.M. An abundantly expressed mucin-like protein from Toxocara canis infective larvae: The precursor of the larval surface coat glycoproteins. Proc. Natl. Acad. Sci. USA 1996, 93, 1665–1670. [Google Scholar] [CrossRef] [Green Version]
- Maizels, R.M.; de Savigny, D.; Ogilvie, B. Characterization of surface and excretory-secretory antigens of Toxocara canis infective larvae. Parasite Immunol. 1984, 6, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Loukas, A.; Doedens, A.; Hintz, M.; Maizels, R.M. Identification of a new C-type lectin, TES-70, secreted by infective larvae of Toxocara canis, which binds to host ligands. Parasitology 2000, 121, 545–554. [Google Scholar] [CrossRef]
- Loukas, A.; Mullin, N.P.; Tetteh, K.K.; Moens, L.; Maizels, R.M. A novel C-type lectin secreted by a tissue-dwelling parasitic nematode. Curr. Biol. 1999, 9, 825–828. [Google Scholar] [CrossRef] [Green Version]
- Schabussova, I.; Amer, H.; van Die, I.; Kosma, P.; Maizels, R.M. O-Methylated glycans from Toxocara are specific targets for antibody binding in human and animal infections. Int. J. Parasitol. 2007, 37, 97–109. [Google Scholar] [CrossRef]
- Długosz, E.; Basałaj, K.; Zawistowska-Deniziak, A. Cytokine production and signalling in human THP-1 macrophages is dependent on Toxocara canis glycans. Parasitol. Res. 2019, 118, 2925–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Długosz, E.; Wasyl, K.; Klockiewicz, M.; Wiśniewski, M. Toxocara canis mucins among other excretory-secretory antigens induce in vitro secretion of cytokines by mouse splenocytes. Parasitol. Res. 2015, 114, 3365–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, E.; Yoshida, Y.; En-Shan, B.; Yamashita, U. Suppression of macrophage interleukin-12 and tumour necrosis factor-alpha production in mice infected with Toxocara canis. Parasite Immunol. 2001, 23, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.A.; Abdel-Aleem, G.A.; Saied, E.M.; Mayah, W.W.; Elatrash, A.M. Biochemical and immunopathological changes in experimental neurotoxocariasis. Mol. Biochem. Parasitol. 2010, 172, 1–8. [Google Scholar] [CrossRef]
- Del Prete, G.F.; de Carli, M.; Mastromauro, C.; Biagiotti, R.; Macchia, D.; Falagiani, P.; Ricci, M.; Romagnani, S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J. Clin. Investig. 1991, 88, 346–350. [Google Scholar] [CrossRef]
- Meghji, M.; Maizels, R.M. Biochemical properties of larval excretory-secretory glycoproteins of the parasitic nematode Toxocara canis. Mol. Biochem. Parasitol. 1986, 18, 155–170. [Google Scholar] [CrossRef]
- van Die, I.; Cummings, R.D. Glycan gimmickry by parasitic helminths: A strategy for modulating the host immune response? Glycobiology 2010, 20, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Mayer, S.; Moeller, R.; Monteiro, J.T.; Ellrott, K.; Josenhans, C.; Lepenies, B. C-Type Lectin Receptor (CLR)–Fc Fusion Proteins As Tools to Screen for Novel CLR/Bacteria Interactions: An Exemplary Study on Preselected Campylobacter jejuni Isolates. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.T.; Schön, K.; Ebbecke, T.; Goethe, R.; Ruland, J.; Baumgärtner, W.; Becker, S.C.; Lepenies, B. The CARD9-Associated C-Type Lectin, Mincle, Recognizes La Crosse Virus (LACV) but Plays a Limited Role in Early Antiviral Responses against LACV. Viruses 2019, 11, 303. [Google Scholar] [CrossRef] [Green Version]
- Raulf, M.K.; Johannssen, T.; Matthiesen, S.; Neumann, K.; Hachenberg, S.; Mayer-Lambertz, S.; Steinbeis, F.; Hegermann, J.; Seeberger, P.H.; Baumgärtner, W.; et al. The C-type lectin receptor CLEC12A recognizes plasmodial hemozoin and contributes to cerebral malaria development. Cell Rep. 2019, 28, 30–38.e35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, M.B.; Urrego, A.J.; Oviedo, Y.; Cooper, P.J.; Pacheco, L.G.; Pinheiro, C.S.; Ferreira, F.; Briza, P.; Alcantara-Neves, N.M. The somatic proteins of Toxocara canis larvae and excretory-secretory products revealed by proteomics. Veter. Parasitol. 2018, 259, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.-B.; Zou, Y.; Zhu, X.-Q.; Liu, G.-H. Toxocara “omics” and the promises it holds for medicine and veterinary medicine. Adv. Parasitol. 2020, 109, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Korhonen, P.K.; Cai, H.; Young, N.D.; Nejsum, P.; von Samson-Himmelstjerna, G.; Boag, P.R.; Tan, P.; Li, Q.; Min, J.; et al. Genetic blueprint of the zoonotic pathogen Toxocara canis. Nat. Commun. 2015, 6, 6145. [Google Scholar] [CrossRef] [Green Version]
- Miyake, Y.; Toyonaga, K.; Mori, D.; Kakuta, S.; Hoshino, Y.; Oyamada, A.; Yamada, H.; Ono, K.; Suyama, M.; Iwakura, Y.; et al. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 2013, 38, 1050–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.-L.; Zhao, X.-Q.; Jiang, C.; You, Y.; Chen, X.-P.; Jiang, Y.-Y.; Jia, X.-M.; Lin, X. C-Type Lectin Receptors Dectin-3 and Dectin-2 Form a Heterodimeric Pattern-Recognition Receptor for Host Defense against Fungal Infection. Immunity 2013, 39, 324–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kooyk, Y.; Ilarregui, J.M.; van Vliet, S.J. Novel insights into the immunomodulatory role of the dendritic cell and mac-rophage-expressed C-type lectin MGL. Immunobiology 2015, 220, 185–192. [Google Scholar] [CrossRef]
- Rodríguez, E.; Carasi, P.; Frigerio, S.; da Costa, V.; van Vliet, S.; Noya, V.; Brossard, N.; van Kooyk, Y.; García-Vallejo, J.J.; Freire, T. Fasciola hepatica Immune Regulates CD11c+ Cells by Interacting with the Macrophage Gal/GalNAc Lectin. Front. Immunol. 2017, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Page, A.; Hamilton, A.; Maizels, R. Toxocara canis: Monoclonal antibodies to carbohydrate epitopes of secreted (TES) antigens localize to different secretion-related structures in infective larvae. Exp. Parasitol. 1992, 75, 56–71. [Google Scholar] [CrossRef]
- Maizels, R.M.; Kennedy, M.W.; Meghji, M.; Robertson, B.D.; Smith, H.V. Shared carbohydrate epitopes on distinct surface and secreted antigens of the parasitic nematode Toxocara canis. J. Immunol. 1987, 139, 207–214. [Google Scholar]
- Maizels, R.; Page, A. Surface associated glycoproteins from Toxocara canis larval parasites. Acta Trop. 1990, 47, 355–364. [Google Scholar] [CrossRef]
- Bowman, D.D. The anatomy of the third-stage larva of Toxocara canis and Toxocara cati. Adv. Parasitol. 2020, 109, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M. Toxocara cati: An underestimated zoonotic agent. Trends Parasitol. 2003, 19, 167–170. [Google Scholar] [CrossRef]
- Zahabiun, F.; Sadjjadi, S.M.; Yunus, M.H.; Rahumatullah, A.; Moghaddam, M.H.; Saidin, S.; Noordin, R. Production of Toxocara cati TES-120 recombinant antigen and comparison with its T. canis homolog for serodiagnosis of toxocariasis. Am. J. Trop. Med. Hyg. 2015, 93, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Gorr, T.A.; Vogel, J. Western blotting revisited: Critical perusal of underappreciated technical issues. Proteom. Clin. Appl. 2015, 9, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Aranzamendi, C.; Sofronic-Milosavljevic, L.; Pinelli, E. Helminths: Immunoregulation and Inflammatory Diseases-Which Side Are Trichinella spp. and Toxocara spp. on? J. Parasitol. Res. 2013, 2013, 329438. [Google Scholar] [CrossRef] [Green Version]
- Junginger, J.; Raue, K.; Wolf, K.; Janecek, E.; Stein, V.M.; Tipold, A.; Günzel-Apel, A.-R.; Strube, C.; Hewicker-Trautwein, M. Zoonotic intestinal helminths interact with the canine immune system by modulating T cell responses and preventing dendritic cell maturation. Sci. Rep. 2017, 7, 10310. [Google Scholar] [CrossRef] [Green Version]
- Carlin, E.P.; Tyungu, D.L. Toxocara: Protecting pets and improving the lives of people. Adv. Parasitol. 2020, 109, 3–16. [Google Scholar] [CrossRef]
- Heuer, L.; Haendel, S.; Beineke, A.; Strube, C. Effects of Toxocara larvae on brain cell survival by in vitro model assessment. Parasitology 2015, 142, 1326–1334. [Google Scholar] [CrossRef]
- Springer, A.; Heuer, L.; Janecek-Erfurth, E.; Beineke, A.; Strube, C. Histopathological characterization of Toxocara canis- and T. cati-induced neurotoxocarosis in the mouse model. Parasitol. Res. 2019, 118, 2591–2600. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyevoich, A.M.; Disher, N.S.; Haro, M.A.; Haas, K.M. A TLR4–TRIF-dependent signaling pathway is required for protective natural tumor-reactive IgM production by B1 cells. Cancer Immunol. Immunother. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Oh-Hora, M.; Yamasaki, S. C-Type Lectin Receptor MCL Facilitates Mincle Expression and Signaling through Complex Formation. J. Immunol. 2015, 194, 5366–5374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaver, E.J.; Kuijk, L.M.; Laan, L.C.; Kringel, H.; van Vliet, S.J.; Bouma, G.; Cummings, R.D.; Kraal, G.; van Die, I. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int. J. Parasitol. 2013, 43, 191–200. [Google Scholar] [CrossRef]
- Meevissen, M.H.; Driessen, N.N.; Smits, H.H.; Versteegh, R.; van Vliet, S.J.; van Kooyk, Y.; Schramm, G.; Deelder, A.M.; Haas, H.; Yazdanbakhsh, M.; et al. Specific glycan elements determine differential binding of individual egg glycoproteins of the human parasite Schistosoma mansoni by host C-type lectin receptors. Int. J. Parasitol. 2012, 42, 269–277. [Google Scholar] [CrossRef]
- van Liempt, E.; van Vliet, S.J.; Engering, A.; García Vallejo, J.J.; Bank, C.M.; Sanchez-Hernandez, M.; van Kooyk, Y.; van Die, I. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 2007, 44, 2605–2615. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; van Liempt, E.; Saeland, E.; Aarnoudse, C.A.; Appelmelk, B.; Irimura, T.; Geijtenbeek, T.B.H.; Blixt, O.; Alvarez, R.; van Die, I.; et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int. Immunol. 2005, 17, 661–669. [Google Scholar] [CrossRef]
- Montero-Barrera, D.; Valderrama-Carvajal, H.; Terrazas, C.A.; Rojas-Hernández, S.; Ledesma-Soto, Y.; Vera-Arias, L.; Carrasco-Yepez, M.; Gómez-García, L.; Martínez-Saucedo, D.; Becerra-Díaz, M.; et al. The Macrophage Galactose-Type Lectin-1 (MGL1) Recognizes Taenia crassiceps Antigens, Triggers Intracellular Signaling, and Is Critical for Resistance to This Infection. BioMed Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Terrazas, C.A.; Alcántara-Hernández, M.; Bonifaz, L.; Terrazas, L.I.; Satoskar, A.R. Helminth-excreted/secreted products are recognized by multiple receptors on DCs to block the TLR response and bias Th2 polarization in a cRAF dependent pathway. FASEB J. 2013, 27, 4547–4560. [Google Scholar] [CrossRef] [Green Version]
- Prasanphanich, N.S.; Mickum, M.L.; Heimburg-Molinaro, J.; Cummings, R.D. Glycoconjugates in Host-Helminth Interactions. Front. Immunol. 2013, 4, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva-Azevedo, L.; Jähne, S.; Hoffmann, C.; Stalder, D.; Heller, M.; Pries, A.R.; Zakrzewicz, A.; Baum, O. Up-regulation of the peroxiredoxin-6 related metabolism of reactive oxygen species in skeletal muscle of mice lacking neuronal nitric oxide synthase. J. Physiol. 2009, 587, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Maglinao, M.; Eriksson, M.; Schlegel, M.K.; Zimmermann, S.; Johannssen, T.; Götze, S.; Seeberger, P.H.; Lepenies, B. A platform to screen for C-type lectin receptor-binding carbohydrates and their potential for cell-specific targeting and immune modulation. J. Control. Release 2014, 175, 36–42. [Google Scholar] [CrossRef]
- Onami, T.M.; Lin, M.-Y.; Page, D.M.; Reynolds, S.A.; Katayama, C.D.; Marth, J.D.; Irimura, T.; Varki, A.; Varki, N.; Hedrick, S.M. Generation of Mice Deficient for Macrophage Galactose- and N-Acetylgalactosamine-Specific Lectin: Limited Role in Lymphoid and Erythroid Homeostasis and Evidence for Multiple Lectins. Mol. Cell. Biol. 2002, 22, 5173–5181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hütter, J.; Eriksson, M.; Johannssen, T.; Klopfleisch, R.; von Smolinski, D.; Gruber, A.D.; Seeberger, P.H.; Lepenies, B. Role of the C-Type Lectin Receptors MCL and DCIR in Experimental Colitis. PLoS ONE 2014, 9, e103281. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Young, J.W. Human dendritic cells: Potent antigen-presenting cells at the crossroads of innate and adaptive im-munity. J. Immunol. 2005, 175, 1373–1381. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raulf, M.-K.; Lepenies, B.; Strube, C. Toxocara canis and Toxocara cati Somatic and Excretory-Secretory Antigens Are Recognised by C-Type Lectin Receptors. Pathogens 2021, 10, 321. https://doi.org/10.3390/pathogens10030321
Raulf M-K, Lepenies B, Strube C. Toxocara canis and Toxocara cati Somatic and Excretory-Secretory Antigens Are Recognised by C-Type Lectin Receptors. Pathogens. 2021; 10(3):321. https://doi.org/10.3390/pathogens10030321
Chicago/Turabian StyleRaulf, Marie-Kristin, Bernd Lepenies, and Christina Strube. 2021. "Toxocara canis and Toxocara cati Somatic and Excretory-Secretory Antigens Are Recognised by C-Type Lectin Receptors" Pathogens 10, no. 3: 321. https://doi.org/10.3390/pathogens10030321
APA StyleRaulf, M. -K., Lepenies, B., & Strube, C. (2021). Toxocara canis and Toxocara cati Somatic and Excretory-Secretory Antigens Are Recognised by C-Type Lectin Receptors. Pathogens, 10(3), 321. https://doi.org/10.3390/pathogens10030321