The Occurrence of Cryptosporidium spp. in Wild-Living Carnivores in Poland—A Question Concerning Its Host Specificity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Specimen Collection
2.2. DNA Extraction
2.3. Molecular Detection and Genotyping of Cryptosporidium spp.
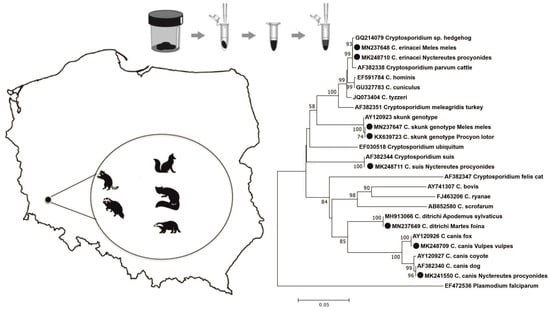
2.4. Sequence and Phylogenetic Analysis
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Ryan, U.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [PubMed]
- Ryan, U.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar]
- Zajączkowska, Ż.; Brutovská, A.B.; Akutko, K.; McEvoy, J.; Sak, B.; Hendrich, A.B.; Łukianowski, B.; Kváč, M.; Kicia, M. Horse-specific Cryptosporidium genotype in human with Crohn’s disease and arthritis. Emerg. Infect. Dis. 2022, 28, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Kváč, M.; Hofmannová, L.; Hlásková, L.; Květoňová, D.; Vítovec, J.; McEvoy, J.; Sak, B. Cryptosporidium erinacei n. sp. (Apicomplexa: Cryptosporidiidae) in hedgehogs. Vet. Parasitol. 2014, 201, 9–17. [Google Scholar] [CrossRef]
- Xiao, L.; Sulaiman, I.M.; Ryan, U.M.; Zhou, L.; Atwill, E.R.; Tischler, M.L.; Zhang, X.; Fayer, R.; Lal, A.A. Host adaptation and host-parasite co-evolution in Cryptosporidium: Implications for taxonomy and public health. Int. J. Parasitol. 2002, 32, 1773–1785. [Google Scholar] [CrossRef]
- Zhou, L.; Fayer, R.; Trout, J.M.; Ryan, U.M.; Schaefer, F.W.; Xiao, L. Genotypes of Cryptosporidium species infecting fur-bearing mammals differ from those of species infecting humans. Appl. Environ. Microbiol. 2004, 70, 7574–7577. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Alderisio, K.A.; Yang, W.; Blancero, L.A.; Kuhne, W.G.; Nadareski, C.A.; Reid, M.; Xiao, L. Cryptosporidium genotypes in wildlife from a New York watershed. Appl. Environ. Microbiol. 2007, 73, 6475–6483. [Google Scholar] [CrossRef] [Green Version]
- Nagano, Y.; Finn, M.B.; Lowery, C.J.; Murphy, T.; Moriarty, J.; Power, E.; Toolan, D.; O’Loughlin, A.; Watabe, M.; McCorry, K.A.; et al. Occurrence of Cryptosporidium parvum and bacterial pathogens in faecal material in the red fox (Vulpes vulpes) population. Vet. Res. Commun. 2007, 31, 559–564. [Google Scholar] [CrossRef]
- Leśniańska, K.; Perec-Matysiak, A.; Hildebrand, J.; Buńkowska-Gawlik, K.; Piróg, A.; Popiołek, M. Cryptosporidium spp. and Enterocytozoon bieneusi in introduced raccoons (Procyon lotor)- first evidence from Poland and Germany. Parasitol. Res. 2016, 115, 4535–4541. [Google Scholar]
- Mateo, M.; de Mingo, M.H.; de Lucio, A.; Morales, L.; Balseiro, A.; Espí, A.; Barral, M.; Lima Barbero, J.F.; Habela, M.Á.; Fernández-García, J.L.; et al. Occurrence and molecular genotyping of Giardia duodenalis and Cryptosporidium spp. in wild mesocarnivores in Spain. Vet. Parasitol. 2017, 235, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Donomoto, T.; Manchanayake, T.; Shibahara, T.; Sasai, K.; Matsubayashi, M. First surveillance and molecular identification of the Cryptosporidium skunk genotype and Cryptosporidium parvum in wild raccoons (Procyon lotor) in Osaka, Japan. Parasitol. Res. 2018, 117, 3669–3674. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Solís, Z.; Meyer-Kayser, E.; Obiegala, A.; Ackermann, F.; Król, N.; Birka, S. Cryptosporidium sp. skunk genotype in wild raccoons (Procyon lotor) naturally infected with Baylisascaris procyonis from Central Germany. Parasitol. Int. 2020, 79, 102159. [Google Scholar] [CrossRef]
- Kváč, M.; Myskova, E.; Holubova, N.; Kellnerova, K.; Kicia, M.; Rajsky, D.; McEvoy, J.; Feng, Y.; Hanzal, V.; Sak, B. Occurrence and genetic diversity of Cryptosporidium spp. in wild foxes, wolves, jackals, and bears in central Europe. Folia Parasitol. 2021, 68, 002. [Google Scholar] [CrossRef]
- Mohammad Rahimi, H.; Soleimani Jevinani, S.; Nemati, S.; Sharifdini, M.; Mirjalali, H.; Zali, M.R. Molecular characterization of Cryptosporidium skunk genotype in raccoons (Procyon lotor) in Iran: Concern for zoonotic transmission. Parasitol. Res. 2022, 121, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tao, W.; Liu, C.; Jiang, Y.; Wan, Q.; Li, Q.; Yang, H.; Lin, Y.; Li, W. First report of Cryptosporidium canis in foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) and identification of several novel subtype families for Cryptosporidium mink genotype in minks (Mustela vison) in China. Infect. Genet. Evol. 2016, 41, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, W.; Wang, J.; Ren, G.; Zhang, W.; Liu, A. Molecular detection and genetic characterizations of Cryptosporidium spp. in farmed foxes, minks, and raccoon dogs in northeastern China. Parasitol. Res. 2018, 117, 169–175. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Y.; Cao, S.; Wu, W.; Zhao, W.; Guo, Y.; Xiao, L.; Feng, Y.; Li, N. Divergent Cryptosporidium species and host-adapted Cryptosporidium canis subtypes in farmed minks, raccoon dogs and foxes in Shandong, China. Front. Cell. Infect. Microbiol. 2022, 12, 980917. [Google Scholar] [CrossRef]
- Xiao, L.; Fayer, R. Molecular characterization of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int. J. Parasitol. 2008, 38, 1239–1255. [Google Scholar] [CrossRef]
- Guo, Y.; Cebelinski, E.; Matusevich, C.; Alderisio, K.A.; Lebbad, M.; McEvoy, J.; Roellig, D.M.; Yang, C.; Feng, Y.; Xiao, L. Subtyping novel zoonotic pathogen Cryptosporidium chipmunk genotype I. J. Clin. Microbiol. 2015, 53, 1648–1654. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Beck, B.; García, F.J.; Gortázar, C. Raccoons in Europe: Disease hazards due to the establishment of an invasive species. Eur. J. Wildl. Res. 2012, 58, 5–15. [Google Scholar] [CrossRef]
- Kauhala, K.; Kowalczyk, R. Invasion of the raccoon dog (Nyctereutes procyonides) in Europe: History of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef]
- Laurimaa, L.; Moks, E.; Soe, E.; Valdmann, H.; Saarma, U. Echinococcus multilocularis and other zoonotic parasites in red foxes in Estonia. Parasitology 2016, 143, 1450–1458. [Google Scholar] [CrossRef]
- Pasanen-Mortensen, M.; Pyykönen, M.; Elmhagen, B. Where lynx prevail, foxes will fail-limitation of a mesopredator in Eurasia. Glob. Ecol. Biogeogr. 2013, 22, 868–877. [Google Scholar] [CrossRef]
- Šálek, M.; Drahníková, L.; Tkadlec, E. Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mammal Rev. 2015, 45, 1–14. [Google Scholar]
- Xiao, L.; Escalante, L.; Yang, C.; Sulaiman, I.; Escalante, A.A.; Montali, R.J.; Fayer, R.; Lal, A.A. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 1999, 65, 1578–1583. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, I.M.; Lal, A.A.; Xiao, L. Molecular phylogeny and evolutionary relationships of Cryptosporidium parasites at the actin locus. J. Parasitol. 2002, 88, 388–394. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Snyder, D.E. Indirect immunofluorescent detection of oocysts of Cryptosporidium parvum in the feces of naturally infected raccoons (Procyon lotor). J. Parasitol. 1988, 74, 1050–1052. [Google Scholar] [CrossRef]
- Yan, W.; Alderisio, K.; Roellig, D.M.; Elwin, K.; Chalmers, R.M.; Yang, F.; Wang, Y.; Feng, Y.; Xiao, L. Subtype analysis of zoonotic pathogen Cryptosporidium skunk genotype. Infect. Genet. Evol. 2017, 55, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Robinson, G.; Elwin, K.; Chalmers, R.M. Unusual Cryptosporidium genotypes in human cases of diarrhea. Emerg. Infect. Dis. 2008, 14, 1800. [Google Scholar] [CrossRef]
- Elwin, K.; Hadfield, S.J.; Robinson, G.; Chalmers, R.M. The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000–2008. Epidemiol. Infect. 2012, 140, 673–683. [Google Scholar] [CrossRef]
- Prediger, J.; Horčičková, M.; Hofmannová, L.; Sak, B.; Ferrari, N.; Mazzamuto, M.V.; Romeo, C.; Wauters, L.A.; McEvoy, J.; Kváč, M. Native and introduced squirrels in Italy host different Cryptosporidium spp. Eur. J. Protistol. 2017, 61, 64–75. [Google Scholar] [CrossRef]
- Barrera, J.P.; Carmena, D.; Rodríguez, E.; Checa, R.; López, A.M.; Fidalgo, L.E.; Gálvez, R.; Marino, V.; Fuentes, I.; Miró, G.; et al. The red fox (Vulpes vulpes) as a potential natural reservoir of human cryptosporidiosis by Cryptosporidium hominis in Northwest Spain. Transbound. Emerg. Dis. 2020, 67, 2172–2182. [Google Scholar] [CrossRef]
- Wandeler, P.; Funk, S.M.; Largiadèr, C.R.; Gloor, S.; Breitenmoser, U. The city-fox phenomenon: Genetic consequences of a recent colonization of urban habitat. Mol. Ecol. 2003, 12, 647–656. [Google Scholar] [CrossRef]
- Kauhala, K.; Holmala, K.; Lammers, W.; Schregel, J. Home ranges and densities of medium-sized carnivores in south-east Finland, with special reference to rabies spread. Acta Theriol. 2006, 51, 1–13. [Google Scholar] [CrossRef]
- Fayer, R.; Santin, M.; Macarisin, D. Cryptosporidium ubiquitum n. sp. in animals and humans. Vet. Parasitol. 2010, 172, 23–32. [Google Scholar] [CrossRef]
- Parsons, M.B.; Travis, D.; Lonsdorf, E.V.; Lipende, I.; Roellig, D.M.; Collins, A.; Kamenya, S.; Zhang, H.; Xiao, L.; Gillespie, T.R. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015, 9, e0003529. [Google Scholar] [CrossRef] [Green Version]
- Horčičková, M.; Čondlová, Š.; Holubová, N.; Sak, B.; Květoňová, D.; Hlásková, L.; Konečný, R.; Sedláček, F.; Clark, M.; Giddings, C.; et al. Diversity of Cryptosporidium in common voles and description of Cryptosporidium alticolis sp. n. and Cryptosporidium microti sp. n. (Apicomplexa: Cryptosporidiidae). Parasitology 2019, 146, 220–233. [Google Scholar] [CrossRef]
- Čondlová, Š.; Horčičková, M.; Sak, B.; Květoňová, D.; Hlásková, L.; Konečný, R.; Stanko, M.; McEvoy, J.; Kváč, M. Cryptosporidium apodemi sp. n. and Cryptosporidium ditrichi sp. n. (Apicomplexa: Cryptosporidiidae) in Apodemus spp. Eur. J. Protistol. 2018, 63, 1–12. [Google Scholar] [CrossRef]
- Čondlová, Š.; Horčičkova, M.; Havrdova, N.; Sak, B.; Hlaskova, L.; Perec-Matysiak, A.; Kicia, M.; McEvoy, J.; Kváč, M. Diversity of Cryptosporidium spp. in Apodemus spp. in Europe. Eur. J. Protistol. 2019, 69, 1–13. [Google Scholar] [CrossRef]
- Hamnes, I.S.; Gierde, B.; Robertson, L.; Vikoren, T.; Handeland, K. Prevalence of Cryptosporidium and Giardia in free-ranging wild cervids in Norway. Vet. Parasitol. 2006, 141, 30–41. [Google Scholar] [CrossRef]
- Hamnes, I.S.; Gierde, B.K.; Forberg, T.; Robertson, L.J. Occurrence of Giardia and Cryptosporidium in Norwegian red foxes (Vulpes vulpes). Vet. Parasitol. 2007, 143, 347–353. [Google Scholar] [CrossRef]
- Perec-Matysiak, A.; Buńkowska-Gawlik, K.; Kváč, M.; Sak, B.; Hildebrand, J.; Leśniańska, K. Diversity of Enterocytozoon bieneusi genotypes among small rodents in southwestern Poland. Vet. Parasitol. 2015, 214, 242–246. [Google Scholar] [CrossRef]
- Sasaki, H.; Kawabata, M. Food habits of the raccoon dog Nyctereutes procyonoides viverrinus in a mountainous area in Japan. J. Mammal. Soc. Jpn. 1994, 19, 1–8. [Google Scholar]
- Takatsuki, S.; Miyaoka, R.; Sugaya, K. A comparison of food habits between Japanese marten and raccoon dog in Western Tokyo with reference to fruit use. Zool. Sci. 2018, 35, 68–74. [Google Scholar] [CrossRef]
- Němejc, K.; Sak, B.; Květoňová, D.; Hanzal, V.; Jeníková, M.; Kváč, M. The first report on Cryptosporidium suis and Cryptosporidium pig genotype II in Eurasian wild boars (Sus scrofa) (Czech Republic). Vet. Parasitol. 2012, 184, 122–125. [Google Scholar] [CrossRef]
- Němejc, K.; Sak, B.; Květoňová, D.; Hanzal, V.; Janiszewski, P.; Forejtek, P.; Rajský, D.; Ravaszová, P.; McEvoy, J.; Kváč, M. Cryptosporidium suis and Cryptosporidium scrofarum in Eurasian wild boars (Sus scrofa) in Central Europe. Vet. Parasitol. 2013, 197, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Castro-Hermida, J.A.; Gracia-Presedo, I.; Gonzalez-Warleta, M.; Mezo, M. Prevalence of Cryptosporidium and Giardia in roe deer (Capreolus capreolus) and wild boars (Sus scrofa) in Galicia (NW, Spain). Vet. Parasitol. 2011, 179, 216–219. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, X.; Chen, J.; Jin, W.; Zhou, X.; Li, N.; Wang, L.; Xiao, L. Occurrence, source, and human infection potential of Cryptosporidium and Giardia spp. in source and tap water in Shanghai, China. Appl. Environ. Microbiol. 2011, 77, 3609–3616. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; An, W.; Chen, Z.; Zhang, D.; Yu, J.; Yang, M. Occurrence and genotypes of Cryptosporidium oocysts in river network of southern-eastern China. Parasitol. Res. 2012, 110, 1701–1709. [Google Scholar] [CrossRef]
- Dyachenko, V.; Kuhnert, Y.; Schmaeschke, R.; Etzold, M.; Pantchev, N.; Daugschies, A. Occurrence and molecular characterization of Cryptosporidium spp. genotypes in European hedgehogs (Erinaceus europaeus L.) in Germany. Parasitology 2010, 137, 205–216. [Google Scholar] [CrossRef]
- Kváč, M.; Květoňová, D.; Sak, B.; Ditrich, O. Cryptosporidium pig genotype II in immunocompetent man. Emerg. Infect. Dis. 2009, 15, 982–983. [Google Scholar] [CrossRef]
- Laatamna, A.E.; Holubova, N.; Sak, B.; Kvac, M. Cryptosporidium meleagridis and C. baileyi (Apicomplexa) in domestic and wild birds in Algeria. Folia Parasitol. 2017, 64, 18. [Google Scholar]
- Ježková, J.; Limpouchová, Z.; Prediger, J.; Holubová, N.; Sak, B.; Konečný, R.; Květoňová, D.; Hlásková, L.; Rost, M.; McEvoy, J.; et al. Cryptosporidium myocastoris n. sp. (Apicomplexa: Cryptosporidiidae), the species adapted to the Nutria (Myocastor coypus). Microorganisms 2021, 9, 813. [Google Scholar] [CrossRef]


| Carnivores/Host Species (Common Name) | No. Examined/No. Positive Samples (%; 95% Confidence Interval) | Genotyping Cryptosporidium spp. Actin Locus (No. of Sequences Samples) | |
|---|---|---|---|
| Procyonidae | Procyon lotor (raccoon) | 65/16 (24.6; 16.2–35.4) | Cryptosporidium skunk genotype (16) |
| Canidae | Nyctereutes procyonoides (raccoon dog) | 87/21 (24.1; 14.5–36.9) | Cryptosporidium canis (dog genotype) (16) Cryptosporidium erinacei (3) Cryptosporidium suis (2) |
| Vulpes vulpes (red fox) | 50/6 (12.0; 4.7–22.8) | Cryptosporidium canis (fox genotype) (3) Cryptosporidium alticolis (2) Cryptosporidium vole genotype II (1) | |
| Mustelidae | Meles meles (badger) | 45/9 (20.0; 9.5–37.1) | Cryptosporidium skunk genotype (5) Cryptosporidium erinacei (4) |
| Martes martes (pine marten) | 24/7 (29.2; 13.9–50.0) | Cryptosporidium ditrichi (7) | |
| Martes foina (beech marten) | 51/15 (29.4; 20.8–39.5) | Cryptosporidium ditrichi (15) | |
| Total | 322/74 (23.0; 18.7–27.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perec-Matysiak, A.; Hildebrand, J.; Popiołek, M.; Buńkowska-Gawlik, K. The Occurrence of Cryptosporidium spp. in Wild-Living Carnivores in Poland—A Question Concerning Its Host Specificity. Pathogens 2023, 12, 198. https://doi.org/10.3390/pathogens12020198
Perec-Matysiak A, Hildebrand J, Popiołek M, Buńkowska-Gawlik K. The Occurrence of Cryptosporidium spp. in Wild-Living Carnivores in Poland—A Question Concerning Its Host Specificity. Pathogens. 2023; 12(2):198. https://doi.org/10.3390/pathogens12020198
Chicago/Turabian StylePerec-Matysiak, Agnieszka, Joanna Hildebrand, Marcin Popiołek, and Katarzyna Buńkowska-Gawlik. 2023. "The Occurrence of Cryptosporidium spp. in Wild-Living Carnivores in Poland—A Question Concerning Its Host Specificity" Pathogens 12, no. 2: 198. https://doi.org/10.3390/pathogens12020198
APA StylePerec-Matysiak, A., Hildebrand, J., Popiołek, M., & Buńkowska-Gawlik, K. (2023). The Occurrence of Cryptosporidium spp. in Wild-Living Carnivores in Poland—A Question Concerning Its Host Specificity. Pathogens, 12(2), 198. https://doi.org/10.3390/pathogens12020198