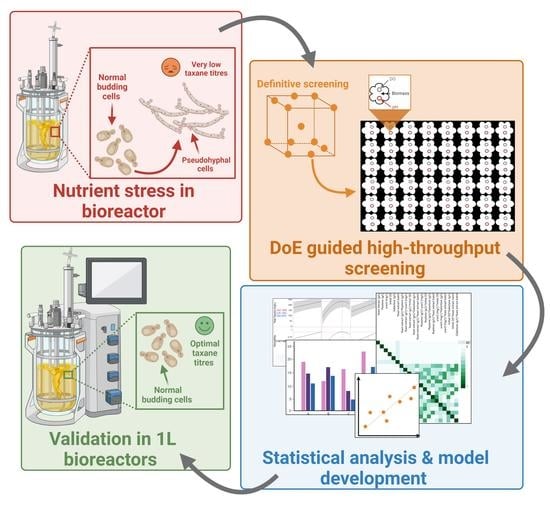
Enhancing Saccharomyces cerevisiae Taxane Biosynthesis and Overcoming Nutritional Stress-Induced Pseudohyphal Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. MiniBio 500 Bioreactor Cultivation
2.3. Microscale Complex Media Optimization
2.4. Bioreactor Cultivation
2.5. Taxane and Metabolite Identification and Quantification
2.6. Statistical Analysis
3. Results and Discussion
3.1. Reproducibility and Factor Determination
3.2. Effect of the Medium on Pseudohyphal Growth in the Minibio 500 Reactor
3.3. Quorum Sensing in S. cerevisiae
3.4. Complex Media Optimization
3.4.1. Microscale Media Optimization Using a Definitive Screening Design
3.4.2. Validation of Optimized Conditions in Highly Instrumented 1L Bioreactors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Malcı, K.; Walls, L.E.; Rios-Solis, L. Multiplex Genome Engineering Methods for Yeast Cell Factory Development. Front. Bioeng. Biotechnol. 2020, 8, 1264. [Google Scholar] [CrossRef]
- Wong, J.; de Rond, T.; d’Espaux, L.; van der Horst, C.; Dev, I.; Rios-Solis, L.; Kirby, J.; Scheller, H.; Keasling, J. High-titer production of lathyrane diterpenoids from sugar by engineered Saccharomyces cerevisiae. Metab. Eng. 2018, 45, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; Rios-Solis, L.; Keasling, J.D. Microbial Production of Isoprenoids. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–24. [Google Scholar]
- Ajikumar, P.K.; Xiao, W.-H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Biggs, B.W.; Lim, C.G.; Sagliani, K.; Shankar, S.; Stephanopoulos, G.; De Mey, M.; Ajikumar, P.K. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 3209–3214. [Google Scholar] [CrossRef] [Green Version]
- Nowrouzi, B.; Li, R.A.; Walls, L.E.; d’Espaux, L.; Malcı, K.; Lungang, L.; Borrego, N.J.; Escalera, A.; Morones-Ramirez, J.R.; Keasling, J.; et al. Enhanced production of taxadiene in Saccharomyces cerevisiae. Microb. Cell Fact. 2020, 19, 200. [Google Scholar] [CrossRef]
- Walls, L.E.; Malcı, K.; Nowrouzi, B.; Li, R.A.; d’Espaux, L.; Wong, J.; Dennis, J.A.; Semião, A.J.C.; Wallace, S.; Martinez, J.L.; et al. Optimizing the biosynthesis of oxygenated and acetylated Taxol precursors in Saccharomyces cerevisiae using advanced bioprocessing strategies. Biotechnol. Bioeng. 2021, 118, 279–293. [Google Scholar] [CrossRef]
- Edgar, S.; Zhou, K.; Qiao, K.; King, J.R.; Simpson, J.H.; Stephanopoulos, G. Mechanistic Insights into Taxadiene Epoxidation by Taxadiene-5α-Hydroxylase. ACS Chem. Biol. 2016, 11, 460–469. [Google Scholar] [CrossRef]
- Dietrich, J.A.; McKee, A.E.; Keasling, J.D. High-Throughput Metabolic Engineering: Advances in Small-Molecule Screening and Selection. Annu. Rev. Biochem. 2010, 79, 563–590. [Google Scholar] [CrossRef]
- Nowrouzi, B.; Rios-Solis, L. Redox metabolism for improving whole-cell P450-catalysed terpenoid biosynthesis. Crit. Rev. Biotechnol. 2021, 1–25. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Chapter 4—Media for industrial fermentations. In Principles of Fermentation Technology, 3rd ed.; Stanbury, P.F., Whitaker, A., Hall, S.J., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 213–272. ISBN 978-0-08-099953-1. [Google Scholar]
- Zhang, J.; Reddy, J.; Buckland, B.; Greasham, R. Toward consistent and productive complex media for industrial fermentations: Studies on yeast extract for a recombinant yeast fermentation process. Biotechnol. Bioeng. 2003, 82, 640–652. [Google Scholar] [CrossRef]
- Potvin, J.; Fonchy, E.; Conway, J.; Champagne, C.P. An automatic turbidimetric method to screen yeast extracts as fermentation nutrient ingredients. J. Microbiol. Methods 1997, 29, 153–160. [Google Scholar] [CrossRef]
- Wilk, P.; Halim, M.; Rios-Solis, L. Recent Advances and Impacts of Microtiter Plate-Based Fermentations in Synthetic Biology and Bioprocess Development. In Fermentation Microbiology and Biotechnology, 4th ed.; El-Mansi, E.M.T., Nielsen, J., Mousdale, D., Carlson, R.P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; p. 14. [Google Scholar]
- Theodoropoulos, C.; Sun, C. 2.45–Bioreactor Models and Modeling Approaches. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M.B.T., Ed.; Pergamon: Oxford, UK, 2019; pp. 663–680. ISBN 978-0-444-64047-5. [Google Scholar]
- Smets, B.; Ghillebert, R.; De Snijder, P.; Binda, M.; Swinnen, E.; De Virgilio, C.; Winderickx, J. Life in the midst of scarcity: Adaptations to nutrient availability in Saccharomyces cerevisiae. Curr. Genet. 2010, 56, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, P.J.; Sprague, G.F., Jr. The Regulation of Filamentous Growth in Yeast. Genetics 2012, 190, 23–49. [Google Scholar] [CrossRef]
- Pothoulakis, G.; Ellis, T. Synthetic gene regulation for independent external induction of the Saccharomyces cerevisiae pseudohyphal growth phenotype. Commun. Biol. 2018, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Kumar, A. An Overview of Autophagy and Yeast Pseudohyphal Growth: Integration of Signaling Pathways during Nitrogen Stress. Cells 2012, 1, 263–283. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.C.; Cutler, N.S.; Heitman, J. Characterization of Alcohol-induced Filamentous Growth inSaccharomyces cerevisiae. Mol. Biol. Cell 2000, 11, 183–199. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Fink, G.R. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 2006, 20, 1150–1161. [Google Scholar] [CrossRef] [Green Version]
- Hogan, D.A. Talking to Themselves: Autoregulation and Quorum Sensing in Fungi. Eukaryot. Cell 2006, 5, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum Sensing in the Dimorphic Fungus Candida albicans Is Mediated by Farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [Green Version]
- Funke, M.; Buchenauer, A.; Schnakenberg, U.; Mokwa, W.; Diederichs, S.; Mertens, A.; Müller, C.; Kensy, F.; Büchs, J. Microfluidic biolector—microfluidic bioprocess control in microtiter plates. Biotechnol. Bioeng. 2010, 107, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.; Goñi-Moreno, A.; Myers, C.; Hecht, A.; de Vicente, M.d.C.; Parco, M.; Schmidt, M.; Timmis, K.; Baldwin, G.; Friedrichs, S.; et al. The long journey towards standards for engineering biosystems. EMBO Rep. 2020, 21, e50521. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Reider Apel, A.; Sachs, D.; Tong, G.J.; D’Espaux, L.; Wehrs, M.; Garber, M.; Nnadi, O.; Mukhopadhyay, A.; Keasling, J.D.; Hillson, N.J.; et al. A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 2016, 45, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.; Nachtsheim, C.J. Definitive Screening Designs with Added Two-Level Categorical Factors. J. Qual. Technol. 2013, 45, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessop-Fabre, M.M.; Sonnenschein, N. Improving Reproducibility in Synthetic Biology. Front. Bioeng. Biotechnol. 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palecek, S.P.; Parikh, A.S.; Huh, J.H.; Kron, S.J. Depression of Saccharomyces cerevisiae invasive growth on non-glucose carbon sources requires the Snf1 kinase. Mol. Microbiol. 2002, 45, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Palková, Z.; Janderová, B.; Gabriel, J.; Zikánová, B.; Pospíŝek, M.; Forstová, J. Ammonia mediates communication between yeast colonies. Nature 1997, 390, 532–536. [Google Scholar] [CrossRef]
- Dickinson, J.R. Filament formation in Saccharomyces cerevisiae—A review. Folia Microbiol. 2008, 53, 3. [Google Scholar] [CrossRef]
- BD Biosciences. BionutrientsTM Technical Manual; BD Biosciences: Miami, FL, USA, 2006. [Google Scholar]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Kebaara, B.W.; Atkin, A.L.; Nickerson, K.W. Regulation of aromatic alcohol production in Candida albicans. Appl. Environ. Microbiol. 2008, 74, 7211–7218. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.A.; Turner, K.J.; James, C.; Fabien, C.; Luisa, D.S.; Dominique, S.; Levin, L.R.; Jochen, B.; Mühlschlegel, F.A. The Quorum-Sensing Molecules Farnesol/Homoserine Lactone and Dodecanol Operate via Distinct Modes of Action in Candida albicans. Eukaryot. Cell 2011, 10, 1034–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, N.M.; Mohan Karuppayil, S. Dual identities for various alcohols in two different yeasts. Mycology 2021, 12, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Garcia, J.H.; Walls, L.E.; Nowrouzi, B.; Ochoa-Villareal, M.; Loake, G.J.; Dimartino, S.; Rios-Solis, L. In situ solid-liquid extraction enhances recovery of taxadiene from engineered S. cerevisiae cell factories. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jones, B.; Nachtsheim, C.J. A Class of Three-Level Designs for Definitive Screening in the Presence of Second-Order Effects. J. Qual. Technol. 2011, 43, 1–15. [Google Scholar] [CrossRef]
- Funke, M.; Diederichs, S.; Kensy, F.; Müller, C.; Büchs, J. The baffled microtiter plate: Increased oxygen transfer and improved online monitoring in small scale fermentations. Biotechnol. Bioeng. 2009, 103, 1118–1128. [Google Scholar] [CrossRef]
- Jouhten, P.; Rintala, E.; Huuskonen, A.; Tamminen, A.; Toivari, M.; Wiebe, M.; Ruohonen, L.; Penttilä, M.; Maaheimo, H. Oxygen dependence of metabolic fluxes and energy generation of Saccharomyces cerevisiae CEN.PK113-1A. BMC Syst. Biol. 2008, 2, 60. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Yan, H.; Li, L.; Zhai, F.; Shang, L.; Yin, Z.; Yuan, Y. Biosynthesis of Taxadiene in Saccharomyces cerevisiae: Selection of Geranylgeranyl Diphosphate Synthase Directed by a Computer-Aided Docking Strategy. PLoS ONE 2014, 9, e109348. [Google Scholar] [CrossRef]
- Egbe, N.E.; Dornelles, T.O.; Paget, C.M.; Castelli, L.M.; Ashe, M.P. Farnesol inhibits translation to limit growth and filamentation in C. albicans and S. cerevisiae. Microb. Cell 2017, 4, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Cowland, T.W.; Maule, D.R. SOME EFFECTS OF AERATION ON THE GROWTH AND METABOLISM OF SACCHAROMYCES CEREVISIAE IN CONTINUOUS CULTURE. J. Inst. Brew. 1966, 72, 480–488. [Google Scholar] [CrossRef]
- Sagwan-Barkdoll, L.; Anterola, A.M. Taxadiene-5α-ol is a minor product of CYP725A4 when expressed in Escherichia coli. Biotechnol. Appl. Biochem. 2018, 65, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Walls, L.E.; Martinez, J.L.; del Rio Chanona, E.A.; Solis, L.R. Definitive screening for accelerated Taxol biosynthetic pathway optimization and scale up in Saccharomyces cerevisiae cell factories. Biotechnol. J. 2021, e2100414. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walls, L.E.; Martinez, J.L.; Rios-Solis, L. Enhancing Saccharomyces cerevisiae Taxane Biosynthesis and Overcoming Nutritional Stress-Induced Pseudohyphal Growth. Microorganisms 2022, 10, 163. https://doi.org/10.3390/microorganisms10010163
Walls LE, Martinez JL, Rios-Solis L. Enhancing Saccharomyces cerevisiae Taxane Biosynthesis and Overcoming Nutritional Stress-Induced Pseudohyphal Growth. Microorganisms. 2022; 10(1):163. https://doi.org/10.3390/microorganisms10010163
Chicago/Turabian StyleWalls, Laura Ellen, José L. Martinez, and Leonardo Rios-Solis. 2022. "Enhancing Saccharomyces cerevisiae Taxane Biosynthesis and Overcoming Nutritional Stress-Induced Pseudohyphal Growth" Microorganisms 10, no. 1: 163. https://doi.org/10.3390/microorganisms10010163
APA StyleWalls, L. E., Martinez, J. L., & Rios-Solis, L. (2022). Enhancing Saccharomyces cerevisiae Taxane Biosynthesis and Overcoming Nutritional Stress-Induced Pseudohyphal Growth. Microorganisms, 10(1), 163. https://doi.org/10.3390/microorganisms10010163