Adult Alphitobius diaperinus Microbial Community during Broiler Production and in Spent Litter after Stockpiling
Abstract
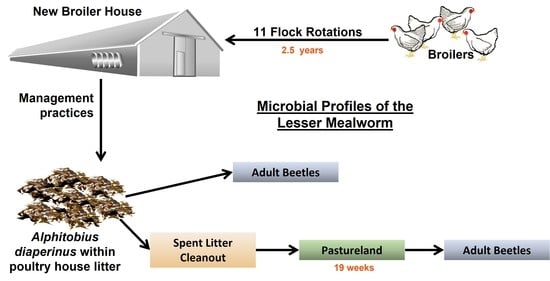
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. In-House Management Practices
2.3. Collections of Beetles within the Poultry House
2.4. Collections of Beetles in Spent Litter
2.5. 16 S rDNA Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. In-House Beetles
3.1.1. In-House Beetles: Alpha Diversity
3.1.2. In-House Beetle: Indicator Species
3.1.3. In-House Beetle: Relative Abundances at the Phylum Level
3.1.4. In-House Beetles: Beta Diversity & Analysis of Molecular Variance (AMOVA)
3.2. Spent Beetles
3.2.1. Spent Beetle: Alpha Diversity
3.2.2. Spent Beetle: Indicator Species
3.2.3. Spent Beetle: Relative Abundances at Phylum Level
3.2.4. Spent Litter: Beta Diversity & AMOVA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Guilhaumon, F.; Missa, O.; Novotny, V.; Ødegaard, F.; Roslin, T.; Schmidl, J.; et al. Arthropod Diversity in a Tropical Forest. Science 2012, 338, 1481–1484. [Google Scholar] [CrossRef] [Green Version]
- Ødegaard, F. How many species of arthropods? Erwin’s estimate revised. Biol. J. Linn. Soc. 2000, 71, 583–597. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, P.; Moran, N.A. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Cucini, C.; Leo, C.; Vitale, M.; Frati, F.; Carapelli, A.; Nardi, F. Bacterial and fungal diversity in the gut of polystyrene-fed Alphitobius diaperinus (Insecta: Coleoptera). Anim. Gene 2020, 17, 200109. [Google Scholar] [CrossRef]
- Leni, G.; Soetemans, L.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Bastiaens, L.; Caligiani, A.; Sforza, S. Protein hydrolysates from Alphitobius diaperinus and Hermetia illucens larvae treated with commercial proteases. J. Insects Food Feed. 2020, 6, 393–404. [Google Scholar] [CrossRef]
- Crippen, T.L.; Sheffield, C.L.; Byrd, J.A.; Esquivel, J.F.; Beier, R.C.; Yeater, K. Poultry litter and the environment: Physiochemical properties of litter and soil during successive flock rotations and after remote site deposition. Sci. Total Environ. 2016, 553, 650–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.A.; McCann, M.A.; Waltman, W.D. Microbiological Survey of Georgia Poultry Litter. J. Appl. Poult. Res. 1998, 7, 90–98. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef]
- Soetemans, L.; Gianotten, N.; Bastiaens, L. Agri-Food Side-Stream Inclusion in The Diet of Alphitobius diaperinus. Part 2: Impact on Larvae Composition. Insects 2020, 11, 190. [Google Scholar] [CrossRef] [Green Version]
- Crippen, T.L.; Poole, T.L. Lesser mealworm on poultry farms: A potential arena for the dissemination of pathogens and anti-microbial resistance. In On-Farm Strategies to Control Foodborne Pathogens; Callaway, T., Edrington, T., Eds.; NOVA Science Publishers: New York, NY, USA, 2012; pp. 233–272. [Google Scholar]
- Arunraj, C.; Sabu, K.; Nirdev, P. Lesser mealworm, Alphitobius diaperinus (Panzer, 1797) (Coleoptera: Tenebrionidae) menace in poultry farms in south India. J. Biopestic. 2013, 6, 84–86. [Google Scholar]
- Gould, F.; Hoffman, G.; Rechenthin, C. Vegetational Areas of Texas. In Texas Agricultural Extension Service Leaflets; Texas Agricultural Experiment Station: College Station, TX, USA, 1960. [Google Scholar]
- Crippen, T.L.; Sheffield, C.L.; Singh, B.; Byrd, J.A.; Beier, R.C.; Anderson, R.C. Poultry litter and the environment: Microbial profile of litter during successive flock rotations and after spreading on pastureland. Sci. Total Environ. 2021, 780, 146413. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Chastain, J.; Camberato, J.; Skewes, P. Poultry Manure Production and Nutrient Content. In CAMM Poultry Training Manual; College of Agriculture, Forestry and Life Sciences, Ed.; Clemson Cooperative Extention, Clemson University: Clemson, SC, USA, 2021. [Google Scholar]
- Martin, E.; Klug, K.; Frischmann, A.; Busse, H.-J.; Kampfer, P.; Jäckel, U. Jeotgalicoccus coquinae sp. nov. and Jeotgalicoccus aerolatus sp. nov., isolated from poultry houses. Int. J. Syst. Evol. Microbiol. 2011, 61, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, S.P.; Kleinhagauer, T.; Jäckel, U.; Klug, K.; Kämpfer, P. Jeotgalicoccus schoeneichii sp. nov. isolated from exhaust air of a pig barn. Int. J. Syst. Evol. Microbiol. 2016, 66, 3503–3508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayres, J.C.; Torrey, G.S.; Salzer, R.H.; Da Silva, G.A.N. Coryneform bacteria in poultry, eggs and meat. J. Appl. Bacteriol. 1966, 29, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Olubade, T.; Nwamo, A.; Sadiku, R. An Outbreak of Corynebacterium diphtheriae infection in broiler chickens in Lagos. Niger. Glob. J. Med. Res. G Vet. Sci. 2016, 16, 7–9. [Google Scholar]
- Barba, M.; Stewart, A.; Passler, T.; Wooldridge, A.; Van Santen, E.; Chamorro, M.; Cattley, R.; Hathcock, T.; Hogsette, J.; Hu, X. Experimental transmission of Corynebacterium pseudotuberculosis Biovar equi in horses by house flies. J. Veter Intern. Med. 2015, 29, 636–643. [Google Scholar] [CrossRef]
- Slover, C.M.; Danziger, L. Lactobacillus: A Review. Clin. Microbiol. Newsl. 2008, 30, 23–27. [Google Scholar] [CrossRef]
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Tao, L.; Yao, H.; Cheng, Q. Genes from a Dietzia sp. for synthesis of C40 and C50 β-cyclic carotenoids. Gene 2007, 386, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, M. Potential applications and emerging trends of species of the genus Dietzia: A review. Ann. Microbiol. 2014, 64, 421–429. [Google Scholar] [CrossRef]
- Haberecht, S.; Bajagai, Y.S.; Moore, R.J.; Van, T.T.H.; Stanley, D. Poultry feeds carry diverse microbial communities that influence chicken intestinal microbiota colonization and maturation. AMB Express 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Chandel, K.; Bora, A.; Veer, V. Isolation and characterization of Dietzia maris from midgut of Aedes albopictus: A suitable candidate for paratransgenesis. Int. J. Mosq. Res. 2015, 2, 7–12. [Google Scholar]
- Wang, L.; Lilburn, M.; Yu, Z. Intestinal microbiota of broiler chickens as affected by litter management regimens. Front. Microbiol. 2016, 7, 593. [Google Scholar] [CrossRef] [Green Version]
- Dumas, M.D.; Polson, S.W.; Ritter, D.; Ravel, J.; Gelb, J.; Morgan, R.; Wommack, K.E. Impacts of poultry house environment on poultry litter bacterial community composition. PLoS ONE 2011, 6, e24785. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.G.; Chen, J.; Chen, Q.H.; Tang, S.K.; Zhang, Y.Q.; He, J.W.; Li, W.J.; Liu, Y.Q. Yaniella soli sp. nov., a new actinobacterium isolated from non-saline forest soil in China. Antonie Van Leeuwenhoek 2010, 98, 395–401. [Google Scholar] [CrossRef]
- Syed, M.A.; Ullah, H.; Tabassum, S.; Fatima, B.; Woodley, T.A.; Ramadan, H.; Jackson, C.R. Staphylococci in poultry intestines: A comparison between farmed and household chickens. Poult. Sci. 2020, 99, 4549–4557. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Souza, S.G.; Campos, G.B.; da Silva, D.C.; Sousa, D.S.; Araújo, S.P.; Ferreira, L.P.; Santos, V.M.; Amorim, A.T.; Santos, A.M.; et al. Isolation, pathogenicity and disinfection of Staphylococcus aureus carried by insects in two public hospitals of Vitória da Conquista, Bahia, Brazil. Braz. J. Infect. Dis. 2014, 18, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.J.; Fanning, G.R.; Huntley-Carter, G.P.; Holmes, B.; Hickman, F.W.; Richard, C.; Brenner, D.J. Kluyvera, a new (re-defined) genus in the family Enterobacteriaceae: Identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescens sp. nov. in clinical specimens. J. Clin. Microbiol. 1981, 13, 919–933. [Google Scholar] [CrossRef] [Green Version]
- Muratoglu, H.; Sezen, K.; Demirbag, Z. Determination and pathogenicity of the bacterial flora associated with the spruce bark beetle, Ips typographus (L.) (Coleoptera: Curculionidae: Scolytinae). Turk. J. Biol. 2011, 35, 9–20. [Google Scholar]
- Rasmussen, M. Aerococci and aerococcal infections. J. Infect. 2013, 66, 467–474. [Google Scholar] [CrossRef]
- Larson, Z.; Subramanyam, B.; Zurek, L.; Herrman, T. Diversity and antibiotic resistance of Enterococci associated with stored-product insects collected from feed mills. J. Stored Prod. Res. 2008, 44, 198–203. [Google Scholar] [CrossRef]
- Turtura, G.; Lorenzelli, P. Gram-positive cocci isolated from slaughtered poultry. Microbiol. Res. 1994, 149, 203–213. [Google Scholar] [CrossRef]
- Madigan, M.; Martinko, J.; Stahl, D.; Clark, D. Brock Biology of Microorganisms, 13th ed.; Pearson Education Inc.: San Francisco, CA, USA, 2012. [Google Scholar]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef] [Green Version]
- Wynants, E.; Crauwels, S.; Verreth, C.; Gianotten, N.; Lievens, B.; Claes, J.; Van Campenhout, L. Microbial dynamics during production of lesser mealworms (Alphitobius diaperinus) for human consumption at industrial scale. Food Microbiol. 2018, 70, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Crippen, T.L.; Sheffield, C.L.; Singh, B.; Byrd, J.A.; Beier, R.C. How management practices within a poultry house during successive flock rotations change the structure of the soil microbiome. Front. Microbiol. 2019, 10, 2100. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.D.; Brown, J.; Jones, D. Brachybacterium faecium gen. nov., sp. nov., a Coryneform bacterium from poultry deep litter. Int. J. Syst. Bacteriol. 1988, 38, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, C.; Karas, P.A.; Vasileiadis, S.; Ligda, P.; Saratsis, A.; Sotiraki, S.; Karpouzas, D.G. Host species determines the composition of the prokaryotic microbiota in Phlebotomus sandflies. Pathogens 2020, 9, 428. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Chen, Z.; Wang, Z.; Lu, Y.; Cheng, D. The divergence in bacterial components associated with Bactrocera dorsalis across developmental stages. Front. Microbiol. 2018, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Wüst, P.K.; Horn, M.A.; Drake, H.L. Clostridiaceae and Enterobacteriaceae as active fermenters in earthworm gut content. ISME J. 2010, 5, 92–106. [Google Scholar] [CrossRef] [Green Version]
- De Cock, M.; Virgilio, M.; Vandamme, P.; Augustinos, A.; Bourtzis, K.; Willems, A.; De Meyer, M. Impact of sample preservation and manipulation on insect gut microbiome profiling. a test case with fruit flies (Diptera, Tephritidae). Front. Microbiol. 2019, 10, 2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilroy, R.; Ravi, A.; Getino, M.; Pursley, I.; Horton, D.L.; Alikhan, N.-F.; Baker, D.; Gharbi, K.; Hall, N.; Watson, M.; et al. Extensive microbial diversity within the chicken gut microbiome revealed by metagenomics and culture. Peer J. 2021, 9, 10941. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Ramalakshmi, P. Studies on efficacy of marine bacterium Salinicoccus roseus pigment for their bioactive potential. Eur. J. Biomed. Pharm. Sci. 2017, 4, 330–334. [Google Scholar]
- Srilekha, V.; Krishna, G.; Srinivas, V.S.; Charya, M.A.S. Antimicrobial evaluation of bioactive pigment from Salinicoccus sp. isolated from Nellore sea coast. Int. J. Biotechnol. Biochem. 2017, 13, 211–217. [Google Scholar]
- Sharifi, Y.; Pourbabaei, A.; Javadi, A.; Abdolmohammad, M.; Saffari, M.; Morovvati, A. Biodegradation of glyphosate herbicide by Salinicoccus spp isolated from Qom Hoze-soltan lake, Iran. Environ. Health Eng. Manag. J. 2015, 2, 31–36. [Google Scholar]
- Cha, Q.-Y.; Zhou, X.-K.; Zhang, X.-F.; Li, M.; Wei, Y.-Q.; Zhang, T.-K.; Qin, S.-C.; Liu, Z.-Y.; Wang, X.-J.; Liu, J.-J.; et al. Luteimonas lumbrici sp. nov., a novel bacterium isolated from wormcast. Int. J. Syst. Evol. Microbiol. 2020, 70, 604–610. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Qian, Y.; Xu, Y.; Wu, H.; Zhang, J.; Huang, H.; Chang, Z. Contributions of thermotolerant bacteria to organic matter degradation under a hyperthermophilic pretreatment process during chicken manure composting. BioResources 2019, 14, 6747–6766. [Google Scholar] [CrossRef]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.; Levy, S.B.; Jackson, R. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conradie, A.T.; Pieterse, E.; Jacobs, K. Application of Paracoccus marcusii as a potential feed additive for laying hens. Poult. Sci. 2018, 97, 986–994. [Google Scholar] [CrossRef]
- Carlson, A.C.; Ingraham, J.L. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl. Environ. Microbiol. 1983, 45, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparic, G.E. The Family Entomoplasmataceae. In The Prokaryotes: Firmicutes and Tenericutes, 4th ed.; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 505–514. [Google Scholar]
- Crippen, T.L.; Sheffield, C.L.; Beier, R.C.; Nisbet, D.J. The horizontal transfer of Salmonella between the lesser mealworm (Alphitobius diaperinus) and poultry manure. Zoonoses Public Health 2018, 65, e23–e33. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Genet. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Dulaurent, A.-M. Assessment of the short-term fertilizer potential of mealworm frass using a pot experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]




| Coverage | Inverse Simpson Index | Shannon Index | Shannon Evenness Index | |||||
|---|---|---|---|---|---|---|---|---|
| In-House | 0.03 | 0.1 | 0.03 | 0.1 | 0.03 | 0.1 | 0.03 | 0.1 |
| Flock 1 | 0.94 | 0.97 | 18.66 | 11.41 | 3.53 | 3.00 | 0.79 | 0.74 |
| Flock 2 | 0.95 | 0.97 | 8.57 | 5.51 | 2.36 | 1.87 | 0.59 | 0.53 |
| Flock 4 | 0.94 | 0.97 | 9.07 | 5.50 | 2.78 | 2.33 | 0.65 | 0.60 |
| Flock 5 | 0.92 | 0.95 | 20.08 | 11.64 | 3.50 | 3.00 | 0.77 | 0.71 |
| Flock 6 | 0.89 | 0.94 | 18.22 | 11.13 | 3.44 | 2.97 | 0.74 | 0.70 |
| Flock 7 | 0.90 | 0.95 | 15.18 | 8.42 | 3.44 | 2.88 | 0.75 | 0.68 |
| Flock 8 | 0.90 | 0.95 | 18.17 | 11.59 | 3.48 | 3.00 | 0.74 | 0.70 |
| Flock 9 | 0.90 | 0.95 | 16.24 | 8.43 | 3.40 | 2.77 | 0.73 | 0.65 |
| Flock 10 | 0.90 | 0.95 | 13.81 | 7.41 | 3.34 | 2.57 | 0.72 | 0.64 |
| Flock 11 | 0.92 | 0.96 | 11.01 | 6.58 | 3.06 | 2.52 | 0.68 | 0.63 |
| Genera | Indicator Group | PBCC * |
|---|---|---|
| Jeotgalicoccus | Flock 1 | 0.839 |
| Corynebacterium | Flock 1 | 0.748 |
| Lactobacillus | Flocks 1, 5 | 0.667 |
| Dietzia | Flock 1 | 0.663 |
| Yaniella | Flocks 6, 7, 8, 9 | 0.660 |
| Staphylococcus | Flocks 2, 4 | 0.649 |
| Kluyvera | Flock 10 | 0.629 |
| Aerococcus | Flock 1 | 0.618 |
| Bacillaceae_unclassified | Flocks 1, 4, 5, 8, 9 | 0.588 |
| Coverage | Inverse Simpson Index | Shannon Index | Shannon Evenness Index | |||||
|---|---|---|---|---|---|---|---|---|
| Field | 0.03 | 0.1 | 0.03 | 0.1 | 0.03 | 0.1 | 0.03 | 0.1 |
| Week 0 | 0.92 | 0.96 | 9.77 | 4.79 | 3.10 | 2.42 | 0.69 | 0.60 |
| Week 1 | 0.91 | 0.96 | 7.55 | 2.96 | 2.83 | 2.01 | 0.64 | 0.51 |
| Week 2 | 0.93 | 0.96 | 2.38 | 2.36 | 1.90 | 1.77 | 0.45 | 0.45 |
| Week 3 | 0.87 | 0.94 | 20.20 | 7.85 | 3.82 | 3.08 | 0.79 | 0.71 |
| Week 5 | 0.64 | 0.79 | 74.89 | 34.32 | 5.00 | 4.33 | 0.89 | 0.83 |
| Week 7 | 0.88 | 0.94 | 17.41 | 9.52 | 3.66 | 2.95 | 0.76 | 0.69 |
| Week 9 | 0.88 | 0.95 | 14.30 | 10.09 | 3.63 | 2.99 | 0.76 | 0.72 |
| Week 11 | 0.86 | 0.93 | 18.55 | 7.58 | 3.85 | 3.06 | 0.77 | 0.69 |
| Week 13 | 0.88 | 0.94 | 23.88 | 10.49 | 3.91 | 3.21 | 0.80 | 0.73 |
| Week 15 | 0.80 | 0.88 | 8.42 | 7.47 | 3.62 | 3.20 | 0.71 | 0.67 |
| Week 17 | 0.86 | 0.93 | 7.07 | 5.98 | 3.34 | 2.75 | 0.68 | 0.63 |
| Week 19 | 0.83 | 0.90 | 5.14 | 4.70 | 3.11 | 2.71 | 0.63 | 0.59 |
| Genera | Indicator Group | PBCC * | Genera | Indicator Group | PBCC * |
|---|---|---|---|---|---|
| Brachybacterium | Weeks 0–5 | 0.747 | Corynebacterium | Weeks 7–19 | 0.786 |
| SMB53 | Weeks 0–5 | 0.715 | Luteimonas | Weeks 7–19 | 0.747 |
| Yaniella | Weeks 0–5 | 0.690 | Gemm.3_unclassified | Weeks 7–19 | 0.732 |
| Gammaproteobacteria_unclassified | Weeks 0–5 | 0.687 | Georgenia | Weeks 7–19 | 0.729 |
| Aerococcaceae_unclassified | Weeks 0–5 | 0.664 | Pseudomonas | Weeks 7–19 | 0.683 |
| Salinicoccus | Weeks 0–5 | 0.661 | Paracoccus | Weeks 7–19 | 0.674 |
| Betaproteobacteria_unclassified | Weeks 0–5 | 0.640 | Rhizobiales_unclassified | Weeks 7–19 | 0.666 |
| Bacillaceae_unclassified | Weeks 0–5 | 0.582 | Alcaligenaceae_unclassified | Weeks 7–19 | 0.663 |
| Bacillales_unclassified | Weeks 0–5 | 0.568 | Brevibacterium | Weeks 7–19 | 0.609 |
| Lactobacillus | Weeks 0–5 | 0.523 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crippen, T.L.; Singh, B.; Anderson, R.C.; Sheffield, C.L. Adult Alphitobius diaperinus Microbial Community during Broiler Production and in Spent Litter after Stockpiling. Microorganisms 2022, 10, 175. https://doi.org/10.3390/microorganisms10010175
Crippen TL, Singh B, Anderson RC, Sheffield CL. Adult Alphitobius diaperinus Microbial Community during Broiler Production and in Spent Litter after Stockpiling. Microorganisms. 2022; 10(1):175. https://doi.org/10.3390/microorganisms10010175
Chicago/Turabian StyleCrippen, Tawni L., Baneshwar Singh, Robin C. Anderson, and Cynthia L. Sheffield. 2022. "Adult Alphitobius diaperinus Microbial Community during Broiler Production and in Spent Litter after Stockpiling" Microorganisms 10, no. 1: 175. https://doi.org/10.3390/microorganisms10010175
APA StyleCrippen, T. L., Singh, B., Anderson, R. C., & Sheffield, C. L. (2022). Adult Alphitobius diaperinus Microbial Community during Broiler Production and in Spent Litter after Stockpiling. Microorganisms, 10(1), 175. https://doi.org/10.3390/microorganisms10010175