Polycyclic Aromatic Hydrocarbon-Degrading Bacteria in Three Different Functional Zones of the Cities of Moscow and Murmansk
Abstract
:1. Introduction
2. Materials and Methods
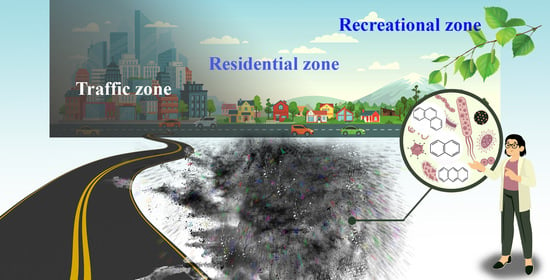
2.1. Climatic Characteristics of Research Areas and Site Description
2.2. Sampling
2.3. Chemical Analysis
2.3.1. Sample Preparation of Road Dust
2.3.2. Sample Preparation of Leaf Dust
2.4. Microbiome Analysis of Dust
2.5. Isolation and Identification of Hydrocarbon-Oxidizing Bacteria
2.5.1. Sample Preparation
2.5.2. Isolation of Hydrocarbon-Oxidizing Bacteria
2.5.3. DNA Manipulations
2.5.4. Taxonomic Identification of Hydrocarbon-Oxidizing Strains
2.6. Statistics
3. Results and Discussion
3.1. Characteristics of Collected Samples from Two Biotopes of Murmansk and Moscow Functional Zones
3.2. Isolation and Characterization of PAH-Degrading Bacteria
3.3. Taxonomic Diversity of Hydrocarbon-Oxidizing Bacteria of Murmansk and Moscow Functional Zones
3.4. Hydrocarbon-Oxidizing Bacteria of Leaf Dust of the Cities’ Functional Zones
3.5. Hydrocarbon-Oxidizing Bacteria of Road Dust of the Cities’ Functional Zones
3.6. PAH of Road and Leaf Surfaces’ Dust of the Cities’ Functional Zones
3.7. Correlation of the Studied Characteristics of Functional Zones Biotopes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110, 451–489. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.; Cincinelli, A.; Martellini, T.; Malik, R.N. A review of PAH exposure from the combustion of biomass fuel and their less surveyed effect on the blood parameters. Environ. Sci. Pollut. Res. 2015, 22, 4076–4098. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAH) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.R.; Karlson, U. Diffuse PAH contamination of surface soils: Environmental occurrence, bioavailability, and microbial degradation. Appl. Microbiol. Biotechnol. 2007, 76, 533–543. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Hu, D.; Wang, X. Polycyclic aromatic hydrocarbons in traffic soil and Pinus needles of Beijing, China. Chem. Spec. Bioavailab. 2011, 23, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Zavgorodnyaya, Y.A.; Chikidova, A.L.; Biryukov, M.V.; Demin, V. Polycyclic aromatic hydrocarbons in atmospheric particulate depositions and urban soils of Moscow, Russia. J. Soils Sediments 2019, 19, 3155–3165. [Google Scholar] [CrossRef]
- Maisto, G.; De Nicola, F.; Prati, M.-V.; Alfani, A. Leaf and soil PAH accumulation in an urban area of the Mediterranean Region (Naples-Italy). Fres. Environ. Bull. 2004, 13, 1263–1268. [Google Scholar]
- Rekefu, S.; Talifu, D.; Gao, B.; Turap, Y.; Maihemuti, M.; Wang, X.; Abulizi, A. Polycyclic Aromatic Hydrocarbons in PM2.5 and PM2.5–10 in Urumqi, China: Temporal Variations, Health Risk, and Sources. Atmosphere 2018, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, A.; Gandolfi, I.; Bestetti, G.; Padoa-Schioppa, E.; Canedoli, C.; Brambilla, D.; Cappelletti, D.; Sebastiani, B.; Federici, E.; Papacchini, M.; et al. Plant–microorganisms interaction promotes removal of air pollutants in Milan (Italy) urban area. J. Hazard. Mater. 2020, 384, 121021. [Google Scholar] [CrossRef] [PubMed]
- Karnchanasest, B.; Satayavibul, A. Orange jasmine leaves as an indicator of atmospheric polycyclic aromatic hydrocarbons. Songklanakarin J. Sci. Technol. 2005, 27, 877–888. [Google Scholar]
- Liang, J.; Fang, H.; Zhang, T.; Wang, X. Polycyclic aromatic hydrocarbons in the leaves of twelve plant species along an urbanization gradient in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2017, 24, 9361–9369. [Google Scholar] [CrossRef]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Bakker, M.I.; Tolls, J.; Kolloffel, C. Deposition of atmospheric semivolatile organic compounds to vegetation. In Persistent, Bioaccumulative, and Toxic Chemicals I: Fate and Exposure; Lipnick, R.L., Hermens, J.L.M., Jones, K.C., Muir, D.C.G., Eds.; American Chemical Society: Washington, DC, USA, 2001; pp. 218–236. [Google Scholar]
- Bohme, F.; Welsch-Pausch, K.; Mclachlan, M.S. Uptake of airborne semivolatile organic compounds in agricultural plants: Field measurements of interspecies variability. Environ. Sci. Technol. 1999, 33, 1805–1813. [Google Scholar] [CrossRef]
- Ivashchenko, K.V.; Korneykova, M.V.; Sazonova, O.I.; Vetrova, A.A.; Ermakova, A.O.; Konstantinov, P.I.; Sotnikova, Y.L.; Soshina, A.S.; Vasileva, M.N.; Vasenev, V.I.; et al. Phylloplane biodiversity and activity in the city at different distances from the traffic pollution source. Plants 2022, 11, 402. [Google Scholar] [CrossRef]
- Denisov, V.V.; Kurbatov, I.A.; Denisova, I.A.; Bondarenko, V.L.; Grachev, B.A.; Gutnev, V.V.; Nagnibeda, B.A. Ecology of the City: Study Guide; Denisov, V.V., Ed.; PBC MarT: Rostov-on-Don, Russia, 2008; 832p. (In Russian) [Google Scholar]
- Ivashchenko, K.V.; Ananyeva, N.; Vasenev, V.I.; Kudeyarov, V.; Valentini, R. Biomass and respiration activity of soil microorganisms in anthropogenically transformed ecosystems (Moscow region). Eurasian Soil Sci. 2014, 47, 892–903. [Google Scholar] [CrossRef]
- Sarzhanov, D.A.; Vasenev, V.I.; Sotnikova, Y.L.; Tembo, A.; Vasenev, I.; Valentini, R. Short-term dynamics and spatial heterogeneity of CO2 emission from the soils of natural and urban ecosystems in the Central Chernozemic Region. Eurasian Soil Sci. 2015, 48, 416–424. [Google Scholar] [CrossRef]
- Lors, C.; Ryngaert, A.; Périé, F.; Diels, L.; Damidot, D. Evolution of bacterial community during bioremediation of PAH in a coal tar contaminated soil. Chemosphere 2010, 81, 1263–1271. [Google Scholar] [CrossRef]
- Martin, F.; Torelli, S.; Le Palier, D.; Barbance, A.; Martin-Laurent, F.; Bru, D.; Geremia, R.; Blake, G.; Jouanneau, Y. Betaproteobacteria dominance and diversity shifts in the bacterial community of a PAH-contaminated soil exposed to phenanthrene. Environ. Pollut. 2012, 162, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Roslund, M.I.; Grönroos, M.; Rantalainen, A.-L.; Jumpponen, A.; Romantschuk, M.; Parajuli, A.; Hyöty, H.; Laitinen, O.; Inkkone, A. Half-lives of PAH and temporal microbiota changes in commonly used urban landscaping materials. Peer J. 2018, 6, 4508. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, A.; Halverson, L.J.; Beattie, G.A. Identification and genetic characterization of phenol-degrading bacteria from leaf microbial communities. Microb. Ecol. 2009, 57, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Yutthammo, C.; Thongthammachat, N.; Pinphanichakarn, P.; Luepromchai, E. Diversity and activity of PAH-degrading bacteria in the phyllosphere of ornamental plants. Microb. Ecol. 2010, 59, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, Y.; Yi, X.; Wang, Z.; Yi, Y.; Huang, T.; Gao, H.; Ma, J. Spatial distribution of atmospheric PAH and their genotoxicity in petrochemical industrialized Lanzhou valley, northwest China. Environ. Sci. Pollut. Res. Int. 2017, 24, 12820–12834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Cheng, S.-P.; Zhu, C.-J.; Sun, S.-L. Microbial PAH-degradation in soil: Degradation pathways and contributing factors. Pedosphere 2006, 16, 555–565. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Wick, L.Y.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, R.; Yu, X.-Z.; Qian, P.-Y.; Wong, M.H. Bacterial communities in PAH contaminated soils at an electronic-waste processing center in China. Ecotoxicology 2010, 19, 96–104. [Google Scholar] [CrossRef]
- Markowicz, A.; Cycoń, M.; Piotrowska-Sege, Z. Microbial community structure and diversity in long-term hydrocarbon and heavy metal contaminated soils. Int. J. Environ. Res. 2016, 10, 321–332. [Google Scholar] [CrossRef]
- Sazonova, O.I.; Sokolov, S.L.; Prisyazhnaya, N.V.; Izmalkova, T.Y.; Kosheleva, I.A.; Boronin, A.M. Epiphytic microorganisms degrading aromatic hydrocarbons from the phyllosphere of urban woody plants. Microbiology 2017, 86, 72–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Cai, J.; Liu, Y.; Hong, L.; Qin, M.; Zheng, M. Atmospheric PAHs in North China: Spatial distribution and sources. Sci. Total Environ. 2016, 565, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cheng, Y.; Qiu, X.; Lin, Y.; Cao, J.; Hu, D. A quantitative assessment of source contributions to fine particulate matter (PM 2.5)-bound polycyclic aromatic hydrocarbons (PAHs) and their nitrated and hydroxylated derivatives in Hong Kong. Environ. Pollut. 2016, 219, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Climate-Data.org. Climate Data for Cities Worldwide. 2021. Available online: https://ru.climate-data.org/ (accessed on 15 February 2021).
- Weather Online Ltd. Meteorological Services. 2021. Available online: https://www.weatheronline.co.uk/ (accessed on 15 February 2021).
- Korneykova, M.V.; Soshina, A.S.; Novikov, A.I.; Ivashchenko, K.V.; Sazonova, O.I.; Slukovskaya, M.V.; Shirokaya, A.A.; Vasenev, V.I.; Vetrova, A.A.; Gavrichkova, O. Microscopic fungi in big cities: Biodiversity, source, and relation to pollution by potentially toxic metals. Atmosphere 2021, 12, 1471. [Google Scholar] [CrossRef]
- US EPA. Priority Pollutant List. 2014. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 3 November 2021).
- Pollegioni, P.; Mattioni, C.; Ristorini, M.; Occhiuto, D.; Canepari, S.; Korneykova, M.V.; Gavrichkova, O. Diversity and source of airborne microbial communities at differential polluted sites of Rome. Atmosphere 2022, 13, 224. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
- Evans, C.G.T.; Herbert, D.; Tempest, D.W. The continuous cultivation of microorganisms: 2. Construction of a chemostat. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 1970; Volume 2, pp. 277–327. [Google Scholar] [CrossRef]
- Mohapatra, B.R.; Broersma, K.; Mazumder, A. Comparison of five rep-PCR genomic fingerprinting methods for differentiation of fecal Escherichia coli from humans, poultry and wild birds. FEMS Microbiol. Lett. 2007, 277, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Pavel, A.B.; Vasile, C.I. PyElph—A software tool for gel images analysis and phylogenetics. BMC Bioinform. 2012, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- BLAST. Available online: http://www.ncbi.nlm.nih.gov/BLAST (accessed on 5 July 2022).
- Pereira Netto, A.D.; Barreto, R.P.; Moreira, J.C.; Arbilla, G. Spatial distribution of polycyclic aromatic hydrocarbons in Terminalia catappa L. (Combretaceae) bark from a selected heavy road traffic area of Rio de Janeiro City, Brazil. J. Hazard. Mater. 2007, 142, 389–396. [Google Scholar] [CrossRef]
- Singh, M.; Jaques, P.A.; Sioutas, C. Size distribution and diurnal characteristics of particle-bound metals in source and receptor site of the Los Angeles Basin. Atm. Environ. 2002, 36, 1675–1689. [Google Scholar] [CrossRef]
- Habe, H.; Omori, T. Genetics of polycyclic aromatic hydrocarbon metabolism in diverse aerobic bacteria. Biosci. Biotechnol. Biochem. 2003, 67, 225–243. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, A.; Halverson, L.J.; Beattie, G.A. Bacterial degradation of airborne phenol in the phyllosphere. Environ. Microbiol. 2007, 9, 383–392. [Google Scholar] [CrossRef]
- Waight, K.; Pinyakong, O.; Luepromchai, E. Degradation of phenanthrene deposited on plant leaves by phyllosphere bacteria. J. Gen. Appl. Microbiol. 2007, 53, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahedi, F.; Dehdari Rad, H.; Goudarzi, G.; Tahmasebi Birgani, Y.; Babaei, A.A.; Ahmadi Angali, K. Polycyclic aromatic hydrocarbons in PM1, PM2.5 and PM10 atmospheric particles: Identification, sources, temporal and spatial variations. J. Environ. Health Sci. Eng. 2021, 19, 851–866. [Google Scholar] [CrossRef]
- Stevens, V.; Thijs, S.; Bongaerts, E.; Nawrot, T.; Marchal, W.; Van Hamme, J.; Vangronsveld, J. Ambient air pollution shapes bacterial and fungal ivy leaf communities. Microorganisms 2021, 9, 2088. [Google Scholar] [CrossRef]
- Marzuki, I.; Asaf, R.; Paena, M.; Athirah, A.; Nisaa, K.; Ahmad, R.; Kamaruddin, M. Anthracene and Pyrene Biodegradation Performance of Marine Sponge Symbiont Bacteria Consortium. Molecules 2021, 26, 6851. [Google Scholar] [CrossRef] [PubMed]
- Premnath, N.; Mohanrasu, K.; Guru Raj Rao, R.; Dinesh, G.H.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic hydrocarbons—Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef]
- Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 Relating to Arsenic, Cadmium, Mercury, Nickel and Polycyclic Aromatic Hydrocarbons in Ambient Air. Available online: http://data.europa.eu/eli/dir/2004/107/2015-09-18 (accessed on 12 January 2022).
- IARC, International Agency for Research on Cancer Monographs on the evaluation of carcinogenic risks to humans. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. Available online: https://www.ncbi.nlm.nih.gov/books/NBK321712/ (accessed on 3 June 2022).
- Jenkins, B.M.; Jones, A.D.; Turn, S.Q.; Williams, R.B. Emission factors for polycyclic aromatic hydrocarbons from biomass burning. Environ. Sci. Technol. 1996, 30, 2462–2469. [Google Scholar] [CrossRef]
- Nascimbene, J.; Tretiach, M.; Corana, F.; Lo Schiavo, F.; Kodnik, D.; Dainese, M.; Mannucci, B. Patterns of traffic polycyclic aromatic hydrocarbon pollution in mountain areas can be revealed by lichen biomonitoring: A case study in the Dolomites (Eastern Italian Alps). Sci. Total Environ. 2014, 475, 90–96. [Google Scholar] [CrossRef]
- Blasco, M.; Domeño, C.; Lopez, P.; Nerin, C. Behavior of different lichen species as biomonitors of air pollution by PAH in natural ecosystems. J. Environ. Monit. 2011, 13, 2588–2596. [Google Scholar] [CrossRef]
- Barberán, A.; Ladau, J.; Leff, J.W.; Pollard, K.S.; Menninger, H.L.; Dunn, R.R.; Fierer, N. Continental-scale distributions of dust-associated bacteria and fungi. Proc. Nat. Acad. Sci. USA 2015, 112, 5756–5761. [Google Scholar] [CrossRef] [Green Version]
- Slukovskii, Z.I. To warm, smoke and pollute: The history of fuel oil in Russia. Pripoda 2020, 7, 3–11. [Google Scholar] [CrossRef]
- Postevaya, M.; Slukovskii, Z. Analysis of atmospheric emissions in Murmansk and their relationship with pollution of urban lakes. Vestnik MGTU 2021, 24, 190–201. [Google Scholar] [CrossRef]
- Fricker, A.M.; Podlesny, D.; Fricke, W.F. What is new and relevant for sequencing-based microbiome research? A mini-review. J. Adv. Res. 2019, 19, 105–112. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Semenov, M.V.; Yakushev, A.V. Activity and Biomass of Soil Microorganisms under Changing Environmental Conditions; Kudeyarov, V.N., Ed.; Association of Scientific Publishing Houses of KMK: Moscow, Russia, 2016; 243p. (In Russian) [Google Scholar]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]





| Genus | Functional Zone | Biotope | Strain | Substrate Specificity * |
|---|---|---|---|---|
| Moscow | ||||
| Acinetobacter | Road dust | M TP4a-1BP | Phn, Sal, BaP, Nah | |
| Traffic | M TP75aPH | Phn, Ant, Sal, BaP, Nah | ||
| Leaf dust | M TL4PH-2 | Phn, Sal, BaP, Nah | ||
| Residential | Road dust | M RP4b-2BP | BP, Nah | |
| Leaf dust | M RL4a-1BP | Phn, Sal, BaP, Nah | ||
| Arthrobacter | Traffic | M TP19A | Phn, Ant, BaP, Nah | |
| Residential | Road dust | M RP23-2PH | Phn, Sal, BaP, Nah | |
| Recreational | M GP3PH | Phn, Ant, Sal, BaP, Nah | ||
| Bacillus | M TP75bPH | Phn, Ant, Sal, BaP, Nah | ||
| M TP12A | Phn, Ant, Sal, BaP, Nah | |||
| M TP24N | Phn, Ant, Sal, BaP, Nah | |||
| M TP4BP | Phn, Sal, BaP, Nah | |||
| Road dust | M TP2BP-3 | Sal, BaP | ||
| Traffic | M TP2BP-2 | BaP | ||
| M TP21A | Phn, Ant, Nah | |||
| M TP5A | Phn, Ant, BaP, Nah | |||
| Leaf dust | M TL38N2 | Phn, Sal, BaP, Nah | ||
| M TL36N-2 | BaP, Nah | |||
| M RP5N | Ant, Sal, BaP, Nah | |||
| M RP1PH | Phn, Sal, BaP, Nah | |||
| M RP60N2 | Phn, Ant, BaP, Nah | |||
| M RP8PH | Phn, Ant, Sal, BaP, Nah | |||
| Road dust | M RP11PH | Phn, Ant, Sal, BaP, Nah | ||
| M RP16-2PH | Phn, Ant, Sal, BaP, Nah | |||
| Residential | M RP16-1BP | Phn, Ant, Sal, BaP, Nah | ||
| M RP22PH | Phn, Ant, Sal, BaP, Nah | |||
| M RP17PH | Phn, Ant, Sal, BaP, Nah | |||
| M RP9PH | Phn, Ant, Sal, Nah | |||
| Leaf dust | M RL23-1PH | Phn, Nah | ||
| M RL4aBP | Phn, BaP, Nah | |||
| Road dust | M GP47PH | Phn, Ant, Sal, Nah | ||
| M GP54PH | Phn, Ant, Sal, BaP, Nah | |||
| Recreational | M GP21N | Phn, Ant, Sal, BaP, Nah | ||
| M GP37N | Phn, Ant, Sal, BaP, Nah | |||
| Leaf dust | M GL3N | Phn, BaP, Nah | ||
| Methylorubrum | M TP31BP-2 | Ant, BaP | ||
| M TP4BP-2 | Ant, Sal, BaP | |||
| Traffic | Road dust | M TP6BP-2 | Ant, Sal, BaP | |
| M TP1BP-2 | Ant, Sal, BaP | |||
| M TP3BP-2 | Sal, BaP | |||
| Leaf dust | M TL33BP-2 | Phn, Sal, BaP, Nah | ||
| Microbacterium | Traffic | Road dust | M RP4b-1BP | Sal, BaP, Nah |
| Residential | Road dust | M GP1a-2BP | BaP, Nah | |
| Micrococcus | Traffic | Leaf dust | M TL5BP-2 | Phn, Sal, BaP, Nah |
| M RL3BP-2 | BaP | |||
| Residential | Leaf dust | M RL1BP-2 | Sal, BaP | |
| M RL5BP-2 | Sal, BaP | |||
| M GP4BP | Ant, Sal, BaP | |||
| Road dust | M GP9BP | Ant, Sal, BaP | ||
| M GP8BP | Phn, Ant, Sal, BaP, Nah | |||
| Recreational | M GL1A-1BP | Sal, BaP | ||
| M GL5N | Sal, Nah | |||
| M GL1PH | Phn, Sal, BaP, Nah | |||
| Leaf dust | M GL4N | Phn, Sal, BaP, Nah | ||
| M GL7PH | Phn, Sal, BaP, Nah | |||
| M GL3aBP | Phn, Sal, BaP, Nah | |||
| M GL6N | Phn, Sal, BaP, Nah | |||
| M GL8N | Phn, Sal, BaP, Nah | |||
| Nocardioides | Recreational | Road dust | M GP7BP | BaP |
| Pantoea | Residential | Road dust | M RP2-1N | Sal, BaP, Nah |
| Paenibacillus | Recreational | Road dust | M RP27PH1 | Phn, Ant, BaP |
| Recreational | Road dust | M GP44PH | Phn, Ant, BaP, Nah | |
| Phyllobacterium | Traffic | Road dust | M TP10BP | Phn, Ant, BaP |
| Pseudarthrobacter | Traffic | Road dust | M TP3PH | Phn, Ant, Sal, BaP, Nah |
| Pseudomonas | Traffic | Road dust | M TP2PH | Phn, Ant, BaP, Nah |
| Leaf dust | M TL29N | Phn, Sal, BaP, Nah | ||
| Recreational | Road dust | M RP6PH | Phn, Ant, Sal, BaP, Nah | |
| M TP11BP | Ant, Sal, BaP, Nah | |||
| M TP1BP | Ant, Sal, BaP, Nah | |||
| M TP5BP | Sal, BaP, Nah | |||
| M TP8BP | Sal, BaP, Nah | |||
| Traffic | Road dust | M TP5PH | Phn, Ant, Sal, BaP, Nah | |
| M TP2BP | Phn, Ant, Sal, BaP, Nah | |||
| M TP9BP | Phn, Ant, Sal, BaP, Nah | |||
| M TP13BP | Phn, Sal, BaP, Nah | |||
| Rhodococcus | M TP14BP | Phn, Ant, BaP, Nah | ||
| M TP7-2BP | Ant, BaP | |||
| M RP1b-1BP | Sal, BaP | |||
| M RP3BP | Ant, Sal, BaP, Nah | |||
| Residential | Road dust | M RP2B-2BP | Ant, Sal, BaP, Nah | |
| M RP2N1 | Sal, Nah | |||
| M RP15PH | Phn, Ant, Sal, BaP, Nah | |||
| Road dust | M GP45bPH | Phn, Ant, Sal, BaP, Nah | ||
| Recreational | M GP35A | Phn, Ant, BaP, Nah | ||
| Leaf dust | M GL2PH | Phn, Sal, BaP, Nah | ||
| Rothia | Traffic | Leaf dust | M TL3PH-2 | Phn, Sal, BaP, Nah |
| Residential | Road dust | M RP4a-2BP | Ant, Sal, BaP, Nah | |
| Stenotrophomonas | Traffic | Road dust | M TP3aBP | Phn, Ant, BaP, Nah |
| Murmansk | ||||
| Traffic | Leaf dust | U TL4BP | Phn, Ant, Sal, BaP, Nah | |
| U TLBP4-1 | Phn, Ant, Sal, BaP, Nah | |||
| Road dust | U RPPH3 | Phn, Ant, Sal, BaP, Nah | ||
| Residential | U RLBP5-2 | Phn, BaP | ||
| Acinetobacter | Leaf dust | U RLBP1-2 | Phn, Ant, BaP | |
| U RLBP3-2 | Ant, BaP | |||
| U GLBP4-2 | Phn, Ant, Sal, BaP, Nah | |||
| Recreational | Leaf dust | U GLN2-2 | Phn, Ant, Sal, BaP, Nah | |
| U GLN3-1 | Sal, BaP, Nah | |||
| Traffic | Leaf dust | U TLA4 | Ant, Sal, BaP, Nah | |
| Residential | Road dust | U RPN1-2 | Phn, Ant, BaP, Nah | |
| Bacillus | Road dust | U GPPH1-2 | Phn, Sal, BaP, Nah | |
| Recreational | U GLBP6 | Phn, Ant, Sal, BaP, Nah | ||
| Leaf dust | U GLPH1-2 | Phn, Ant, Sal, BaP, Nah | ||
| U GLBP4 | Phn, Ant, Sal, BaP | |||
| Brevundimonas | Traffic | Leaf dust | U TLBP4-1 | Phn, Ant, Sal, BaP, Nah |
| U RLBP5-2 | Ant, BaP | |||
| Residential | Leaf dust | U RLBP1-2 | Phn, Ant, BaP | |
| U RLBP3-2 | Ant, BaP | |||
| Cellulomonas | Residential | Road dust | U RPBP1 | Ant, BaP, Nah |
| Curtobacterium | Residential | Road dust | U RPN1-1 | Phn, Sal, Nah |
| Deinococcus | Traffic | Road dust | U TPPH2 | Phn, Ant, Sal, BaP |
| U TPA1 | Phn, Ant, Sal, BaP, Nah | |||
| Dermacoccus | Traffic | Road dust | U GPN1 | Phn, Ant, BaP, Nah |
| U GPN3 | Phn, Ant, BaP, Nah | |||
| Exiguobacterium | Traffic | Leaf dust | U TL1BP | Phn, Ant, BaP |
| Gordonia | Traffic | Road dust | U TPA4-2 | Phn, Ant, BaP |
| Kocuria | Residential | Leaf dust | U RLPH3 | Phn, Sal, Nah |
| Leifsonia | Recreational | Road dust | U GPA3 | Ant, Sal, BaP |
| Enterobacter | Residential | Leaf dust | U RLBP5 | Phn, Ant, Sal, BaP |
| Methylobacterium | Traffic | Leaf dust | U TLPH5 | Phn, Ant |
| U TLPH2-2, | Phn, Sal, BaP, Nah | |||
| U TLPH5-1 | Phn, Sal, BaP, Nah | |||
| Traffic | Leaf dust | U TLBP5-2 | Phn, Ant, Sal, BaP, Nah | |
| U TLPH6-2 | Phn, Ant, Sal, BaP | |||
| Microbacterium | U TLPH6 | Phn, Ant, Sal, BaP | ||
| U RPN2 | Sal, BaP, Nah | |||
| Road dust | U RPPH4 | Phn, BaP, Nah | ||
| U RPBP3 | Ant, Sal, BaP, Nah | |||
| Residential | U RLBP1 | BaP | ||
| Leaf dust | U RLN2 | Phn, Sal, Nah | ||
| U RLBP2 | BaP, Nah | |||
| U RLA2 | Ant | |||
| Recreational | Road dust | U GPBP5 | Phn, Ant, BaP, Nah | |
| U GPPH4 | Phn, Ant, Sal, BaP | |||
| U TLBP2 | Phn, Ant, Sal, BaP, Nah | |||
| U TLPH4-1 | Phn, Ant, Sal, BaP, Nah | |||
| Traffic | Leaf dust | U TLPH8 | Phn, Ant, Sal, BaP, Nah | |
| U TL6BP | Phn, Ant, Sal, BaP, Nah | |||
| U TL7BP | Phn, Ant, Sal, BaP, Nah | |||
| Road dust | U RPPH3-2 | Phn, Ant, Sal, BaP, Nah | ||
| Micrococcus | Residential | U RLPH1 | Phn, Ant, Sal, BaP, Nah | |
| Leaf dust | U RLN3 | Ant, BaP, Nah | ||
| U RLBP1 | Ant, Sal, BaP, Nah | |||
| Road dust | U GPA3-2, | Ant, Sal, BaP, Nah | ||
| U GPPH4-2 | Phn, Sal, BaP, Nah | |||
| Recreational | U GLN2 | Phn, Ant, Sal, BaP, Nah | ||
| Leaf dust | U GLN2-1 | Phn, Ant, Sal, BaP, Nah | ||
| U GLN3 | Phn, Ant, Sal, BaP, Nah | |||
| U GLPH1 | Phn, Ant, BaP | |||
| Moraxella | Recreational | Leaf dust | U GLA1 | Phn, Ant, BaP |
| Nocardioides | Recreational | Road dust | U GP6BP | BaP, Nah |
| Traffic | Leaf dust | U TLA2 | Ant, Sal, BaP, Nah | |
| Paenibacillus | U TLPH1 | Phn, Ant, Sal, BaP, Nah | ||
| Residential | Road dust | U RLPH1-1 | Phn, Ant, Sal, BaP, Nah | |
| Recreational | Leaf dust | U GLN7-2 | Ant, Sal, BaP, Nah | |
| Traffic | Leaf dust | U TLPH1-1 | Phn, BaP, Nah | |
| U TLPH2 | Phn, Ant, BaP, Nah | |||
| U RPPH2, | Phn, Sal, BaP, Nah | |||
| Pseudomonas | U RPPH1 | Phn, Sal, BaP, Nah | ||
| Residential | Road dust | U RPPH4-2 | Phn, BaP, Nah | |
| U RPN1 | Ant, Sal, Nah | |||
| U RPN3 | Sal, BaP, Nah | |||
| Rahnella | Recreational | Leaf dust | U GLA2 | Ant, Nah |
| Rhizobium | Recreational | Leaf dust | U GLPH2-2 | Phn, Ant |
| U TPPH5 | Phn, Sal, BaP, Nah | |||
| Traffic | Road dust | U TPN1 | Ant, Sal, BaP, Nah | |
| U TPPH7 | Phn, Ant, Sal, BaP, Nah | |||
| Leaf dust | U TLN2 | Ant, Sal, BaP, Nah | ||
| U RPPH2-2 | Phn, Ant, Sal, BaP, Nah | |||
| Rhodococcus | Residential | U RPPH1-2 | Phn, Ant, Sal, BaP, Nah | |
| Leaf dust | U RLBP3 | Phn, Ant, Sal, BaP, Nah | ||
| U GPBP1 | Phn, BaP, Nah | |||
| Recreational | Road dust | U GPBP3 | Ant, BaP, Nah | |
| U GPN2 | Phn, Ant, Sal, BaP, Nah | |||
| Rothia | Road dust | U TPA4 | Phn, Ant, Sal, BaP, Nah | |
| Traffic | Leaf dust | U TLBP5-1 | Phn, Ant, BaP, Nah | |
| U TL3-1BP | Phn, Ant, BaP, Nah | |||
| Residential | Leaf dust | U RLN1 | Phn, Ant, BaP, Nah | |
| U RLBP1-1 | Phn, Ant, BaP, Nah | |||
| U GLPH2 | Phn, Ant, BaP, Nah | |||
| Recreational | Leaf dust | U GLBP5 | Phn, Ant, BaP, Nah | |
| U GLN1 | Ant, BaP, Nah | |||
| Shinella | Traffic | Leaf dust | U TL3BP | BaP |
| Staphylococcus | Traffic | Leaf dust | U TLPH1-2 | Phn, Sal |
| U TLA3-2 | Ant, Sal, BaP | |||
| Stenotrophomonas | Traffic | Leaf dust | U TLBP4 | Phn, BaP |
| Streptomyces | Recreational | Road dust | U GPA1 | Phn, Ant, Sal, BaP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazonova, O.I.; Gavrichkova, O.; Ivanova, A.A.; Petrikov, K.V.; Streletskii, R.A.; Sarzhanov, D.A.; Korneykova, M.V.; Novikov, A.I.; Vasenev, V.I.; Ivashchenko, K.V.; et al. Polycyclic Aromatic Hydrocarbon-Degrading Bacteria in Three Different Functional Zones of the Cities of Moscow and Murmansk. Microorganisms 2022, 10, 1979. https://doi.org/10.3390/microorganisms10101979
Sazonova OI, Gavrichkova O, Ivanova AA, Petrikov KV, Streletskii RA, Sarzhanov DA, Korneykova MV, Novikov AI, Vasenev VI, Ivashchenko KV, et al. Polycyclic Aromatic Hydrocarbon-Degrading Bacteria in Three Different Functional Zones of the Cities of Moscow and Murmansk. Microorganisms. 2022; 10(10):1979. https://doi.org/10.3390/microorganisms10101979
Chicago/Turabian StyleSazonova, Olesya I., Olga Gavrichkova, Anastasia A. Ivanova, Kirill V. Petrikov, Rostislav A. Streletskii, Dmitriy A. Sarzhanov, Maria V. Korneykova, Andrey I. Novikov, Viacheslav I. Vasenev, Kristina V. Ivashchenko, and et al. 2022. "Polycyclic Aromatic Hydrocarbon-Degrading Bacteria in Three Different Functional Zones of the Cities of Moscow and Murmansk" Microorganisms 10, no. 10: 1979. https://doi.org/10.3390/microorganisms10101979
APA StyleSazonova, O. I., Gavrichkova, O., Ivanova, A. A., Petrikov, K. V., Streletskii, R. A., Sarzhanov, D. A., Korneykova, M. V., Novikov, A. I., Vasenev, V. I., Ivashchenko, K. V., Slukovskaya, M. V., & Vetrova, A. A. (2022). Polycyclic Aromatic Hydrocarbon-Degrading Bacteria in Three Different Functional Zones of the Cities of Moscow and Murmansk. Microorganisms, 10(10), 1979. https://doi.org/10.3390/microorganisms10101979