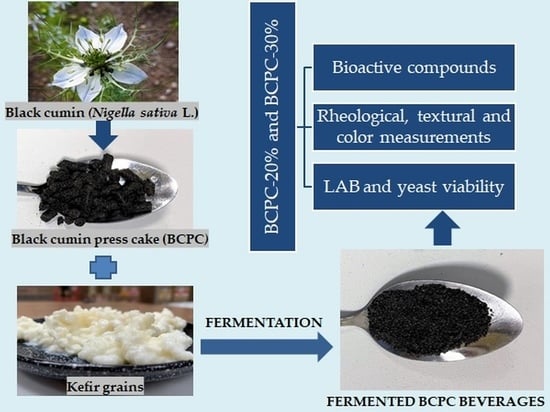
Black Cumin (Nigella sativa L.) Seed Press Cake as a Novel Material for the Development of New Non-Dairy Beverage Fermented with Kefir Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fermentation and Preparation of the Samples
2.3. Microbiological Analyses, pH Determination, and Total Solids Content (TSC)
2.4. Preparation of Extracts
2.5. Determination of Total Polyphenolic Content (TPC), Total Flavonoids Content (TFC), Reducing Sugars Content (RSC), and Total Free Amino Acids Level (TFAAL)
2.6. Texture Analysis and Rheological Measurement
2.7. Color Measurements
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Lactic Acid Bacteria (LAB) and Yeasts Viability during Storage
3.2. pH and Reducing Sugars
3.3. The Changes of Total Free Amino Acid Level (TFAAL), Total Polyphenolics Content (TPC), and Total Flavonoids Content (TFC)
3.4. Color Changes
3.5. Rheological and Textural Changes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of Innovative Food Processing Technologies on the Physicochemical and Nutritional Properties and Quality of Non-Dairy Plant-Based Beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseed oil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Hassanien, M.F.R.; Assiri, A.M.A.; Alzohairy, A.M.; Oraby, H.F. Health-Promoting Value and Food Applications of Black Cumin Essential Oil: An Overview. J. Food Sci. Technol. 2015, 52, 6136–6142. [Google Scholar] [CrossRef]
- Coşkun, Ö.; Çağlar, A.F.; Çakır, B.; Gülseren, İ. Influence of Maillard reaction conditions and solvent extraction on the surface activity and foaming characteristics of black cumin protein concentrates. J. Food Sci. Technol. 2021, 58, 4323–4332. [Google Scholar] [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) Seeds: Traditional Uses, Chemical Constituents, and Nutraceutical Effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Zaky, A.A.; Shim, J.H.; Abd El-Aty, A.M. A Review on Extraction, Characterization, and Applications of Bioactive Peptides From Pressed Black Cumin Seed Cake. Front. Nutr. 2021, 8, 609. [Google Scholar] [CrossRef]
- Różyło, R.; Piekut, J.; Wójcik, M.; Kozłowicz, K.; Smolewska, M.; Krajewska, M.; Szmigielski, M.; Bourekoua, H. Black Cumin Pressing Waste Material as a Functional Additive for Starch Bread. Materials 2021, 14, 4560. [Google Scholar] [CrossRef]
- Rostami, H.; Hamedi, H.; Ghaderi, M. Viability of Commercial Probiotic Cultures in Cottage Cheese Containing Black Cumin Seed. J. Food Meas. Charact. 2018, 12, 1648–1653. [Google Scholar] [CrossRef]
- Abu-Jdayil, B. Rheology of Milled Black Cumin (Nigella sativa L.). Rheol. Acta 2002, 41, 441–447. [Google Scholar] [CrossRef]
- Şen, N.; Kar, Y.; Tekeli, Y. Antioxidant Activities of Black Cumin (Nigella sativa L.) Seeds Cultivating in Different Regions of Turkey. J. Food Biochem. 2010, 34, 105–119. [Google Scholar] [CrossRef]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of Lab-Fermented Foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Heath–Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Neubauer, P. A Big World in Small Grain: A Review of Natural Milk Kefir Starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kwiatkowski, P.; Bartkowiak, A.; Gefrom, A.; Sienkiewicz, M. The Effect of Fermentation with Kefir Grains on the Physicochemical and Antioxidant Properties of Beverages from Blue Lupin (Lupinus angustifolius L.) seeds. Molecules 2020, 25, 5791. [Google Scholar] [CrossRef]
- Farag, M.A.; Jomaa, S.A.; El-Aida, A.; El-Seedi, H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Tong, T.; Liu, Y.J.; Kang, J.; Zhang, C.M.; Kang, S.G. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, X.M.; Guo, S.D. Rheological, Texture and Sensory Properties of Low-Fat Mayonnaise with Different Fat Mimetics. LWT Food Sci. Technol. 2007, 40, 946–954. [Google Scholar] [CrossRef]
- Kazazi, H.; Khodaiyan, F.; Rezaei, K.; Pishvaei, M.; Mohammadifar, M.A.; Moieni, S. Rheology and Microstructure of Kefiran and Whey Protein Mixed Gels. J. Food Sci. Technol. 2017, 54, 1168–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkinen, O.E.; Uniacke-Lowe, T.; O’Mahony, J.A.; Arendt, E.K. Physicochemical and Acid Gelation Properties of Commercial UHT-Treated Plant-Based Milk Substitutes and Lactose Free Bovine Milk. Food Chem. 2015, 168, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with Broad Antifungal Activity: A Promising Approach to Increase Safety and Shelf-Life of Cereal-Based Products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef]
- FAO. Standard for Fermented Milks; CXS 243-2003, Revised; Food Agriculture Organization (FAO): Rome, Italy, 2018. [Google Scholar]
- Arici, M.; Sagdic, O.; Gecgel, U. Antibacterial Effect of Turkish Black Cumin (Nigella sativa L.) oils. Grasas Y Aceites 2005, 56, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Georgescu, M.; Tăpăloagă, P.R.; Tăpăloagă, D.; Furnaris, F.; Ginghină, O.; Negrei, C.; Giuglea, C.; Bălălău, C.; Ștefănescu, E.; Popescu, I.A.; et al. Evaluation of Antimicrobial Potential of Nigella Sativa Oil in a Model Food Matrix. Farmacia 2018, 66, 1028–1036. [Google Scholar] [CrossRef]
- Monsoor, M.A. Effect of Drying Methods on the Functional Properties of Soy Hull Pectin. Carbohydr. Polym. 2005, 61, 362–367. [Google Scholar] [CrossRef]
- Nissen, L.; di Carlo, E.; Gianotti, A. Prebiotic Potential of Hemp Blended Drinks Fermented by Probiotics. Food Res. Int. 2020, 131, 109029. [Google Scholar] [CrossRef]
- Lim, X.X.; Koh, W.Y.; Uthumporn, U.; Maizura, M.; Wan Rosli, W.I. The Development of Legume-Based Yogurt by Using Water Kefir as Starter Culture. Int. Food Res. J. 2019, 26, 1219–1228. [Google Scholar]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C.G. Fermented Cereal-Based products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Toma, C.C.; Olah, N.K.; Vlase, L.; Mogoşan, C.; Mocan, A. Comparative Studies on Polyphenolic Composition, Antioxidant and Diuretic Effects of Nigella sativa L. (Black Cumin) and Nigella damascena L. (Lady-in-a-Mist) Seeds. Molecules 2015, 20, 9560–9574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant Activity and Phenolic Content of Phenolic Rich Fractions Obtained from Black Cumin (Nigella sativa) Seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Xia, N.; He, Z.Y.; Kang, W.Y. Total phenolic and flavoniod content and antioxidant properties of Nigella sativa L. seeds. Curr. Top. Nutraceut. Res. 2018, 16, 147–154. [Google Scholar]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Quezada, N.; Cherian, G. Lipid Characterization and Antioxidant Status of the Seeds and Meals of Camelina sativa and Flax. Eur. J. Lipid Sci. Technol. 2012, 114, 974–982. [Google Scholar] [CrossRef]
- Salachna, P.; Pietrak, A.; Łopusiewicz, Ł. Antioxidant Potential of Flower Extracts from Centaurea spp. Depends on Their Content of Phenolics, Flavonoids and Free Amino Acids. Molecules 2021, 26, 7465. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent. Polymers 2020, 12, 2207. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S. Effects of Protein Concentration and Oil-Phase Volume Fraction on the Stability and Rheology of Menhaden Oil-in-Water Emulsions Stabilized by Whey Protein Isolate with Xanthan Gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Tan, G.B.; Liu, S.H.; Wang, D.G.; Zhang, S.W. Spatio-Temporal Structure in Wax-Oil Gel Scraping at a Soft Tribological Contact. Tribol. Int. 2015, 88, 236–251. [Google Scholar] [CrossRef]
- Dimitreli, G.; Antoniou, K.D. Effect of Incubation Temperature and Caseinates on the Rheological Behaviour of Kefir. Procedia Food Sci. 2011, 1, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Gul, O.; Atalar, I.; Mortas, M.; Dervisoglu, M. Rheological, Textural, Colour and Sensorial Properties of Kefir Produced with Buffalo Milk Using Kefir Grains and Starter Culture: A Comparison with Cows’ Milk Kefir. Int. J. Dairy Technol. 2018, 71, 73–80. [Google Scholar] [CrossRef]
- Glibowski, P.; Kowalska, A. Rheological, Texture and Sensory Properties of Kefir with High Performance and Native Inulin. J. Food Eng. 2012, 111, 299–304. [Google Scholar] [CrossRef]
- Sady, M.; Domagała, J.; Najgebauer-Lejko, D.; Grega, T. Effect of Whey Protein Concentrate Addition on Texture and Rheological Properties of Kefir Produced from Skimmed Milk. Biotechnol. Anim. Husb. 2009, 25, 763–771. [Google Scholar]


| Time of Storage (Days) | |||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| pH | |||||||
| BCPC-20% | 5.93 ± 0.02 Aa | 5.60 ± 0.01 Ba | 5.85 ± 0.01 Ca | 5.62 ± 0.04 Da | 5.73 ± 0.07 Ea | 5.76 ± 0.01 Fa | 5.73 ± 0.03 Ga |
| BCPC-30% | 5.89 ± 0.01Ab | 5.74 ± 0.03 Bb | 5.88 ± 0.01 Ab | 5.78 ± 0.01 Cb | 5.86 ± 0.01 Db | 5.61 ± 0.02 Eb | 5.76 ± 0.01 Fa |
| RSC (mg /mL) | |||||||
| BCPC-20% | 69.19 ± 1.33 Aa | 31.94 ± 0.00 Ba | 30.53 ± 0.67 BCa | 27.37 ± 0.38 Ca | 29.45 ± 0.09 BCa | 22.19 ± 0.09 Da | 29.11 ± 0.38 BCa |
| BCPC-30% | 83.85 ± 7.42 Ab | 31.20 ± 0.28 BCa | 27.97 ± 0.09 BCa | 27.30 ± 0.66 Ca | 27.16 ± 0.66 Ca | 21.92 ± 0.47 Da | 18.49 ± 0.19 Db |
| TSC (%) | |||||||
| BCPC-20% | 19.90 ± 0.72 Aa | 15.66 ± 4.94 Ba | 18.72 ± 0.14 BCa | 18.52 ± 0.08 BCa | 18.14 ± 0.20 BCa | 19.40 ± 0.11 Ca | 19.63 ± 2.69 Ca |
| BCPC-30% | 28.50 ± 0.76 Ab | 27.98 ± 0.56 Bb | 26.71 ± 0.07 BCb | 26.39 ± 0.06 BCb | 24.98 ± 0.94 BCb | 26.57 ± 0.22 BCb | 23.68 ± 4.62 Cb |
| Time of Storage (Days) | |||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| TFAAL (mg Gly/mL) | |||||||
| BCPC-20% | 1.19 ± 0.05 ABCa | 1.21 ± 0.02 ABa | 1.01 ± 0.06 Da | 0.73 ± 0.01 Ea | 1.16 ± 0.05 BCa | 1.23 ± 0.06 Aa | 0.94 ± 0.02 Fa |
| BCPC-30% | 1.03 ± 0.03 Ab | 1.00 ± 0.01 Ab | 0.81 ± 0.03 Bb | 1.05 ± 0.04 Ab | 1.22 ± 0.01 Ca | 1.21 ± 0.00 Db | 1.43 ± 0.00 Eb |
| TPC (mg GAE/mL) | |||||||
| BCPC-20% | 6.10 ± 0.42 ABa | 6.25 ± 1.20 ABa | 6.48 ± 0.82 ABa | 5.53 ± 0.85 Aa | 6.82 ± 0.66 ABa | 6.88 ± 0.76 BCa | 6.69 ± 0.39 ABa |
| BCPC-30% | 5.96 ± 0.73 ABb | 6.15 ± 0.49 ABa | 6.22 ± 0.03 ABa | 6.53 ± 0.11 ABa | 6.46 ± 0.14 ABa | 5.54 ± 0.00 Ab | 8.15 ± 0.00 Cb |
| TFC (mg QE/mL) | |||||||
| BCPC-20% | 0.35 ± 0.07 ABa | 1.01 ± 0.22 DEa | 0.69 ± 0.04 ABCDa | 0.31 ± 0.60 Aa | 0.65 ± 0.06 ABCDa | 0.31 ± 0.02 Aa | 0.54 ± 0.06 ABCa |
| BCPC-30% | 0.85 ± 0.56 ABb | 0.86 ± 0.21 ABa | 0.72 ± 0.0 ABCDa | 1.02 ± 0.09 AEb | 1.38 ± 0.15 Eb | 0.75 ± 0.15 ABCb | 0.75 ± 0.22 ABCa |
| Time of Storage (Days) | |||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| L* | |||||||
| BCPC-20% | 21.29 ± 0.12 Aa | 20.45 ± 0.07 Ba | 22.00 ± 0.07 Ca | 22.19 ± 0.07 Da | 20.31 ± 0.02 Ea | 22.07 ± 0.01 Fa | 19.52 ± 0.02 Gb |
| BCPC-30% | 17.87 ± 0.02 Ab | 17.72 ± 0.05 Bb | 19.65 ± 0.02 Cb | 18.89 ± 0.11 Db | 17.78 ± 0.03 Eb | 19.72 ± 0.04 Fb | 19.52 ± 0.03 Eb |
| a* | |||||||
| BCPC-20% | 2.18 ± 0.03 Aa | 2.42 ± 0.03 Ba | 2.45 ± 0.03 Ca | 2.45 ± 0.03 Ca | 2.41 ± 0.03 Ba | 2.40 ± 0.03 Ba | 1.95 ± 0.05 Da |
| BCPC-30% | 1.87 ± 0.02 Ab | 2.05 ± 0.06 Bb | 1.96 ± 0.03 Cb | 1.89 ± 0.02 Ab | 1.76 ± 0.05 Db | 1.97 ± 0.05 Cb | 1.52 ± 0.02 Eb |
| b* | |||||||
| BCPC-20% | 5.92 ± 0.06 Aa | 7.63 ± 0.04 Ba | 8.08 ± 0.04 Ca | 8.09 ± 0.05 Ca | 7.17 ± 0.05 Da | 7.58 ± 0.03 Ea | 5.37 ± 0.08 Fa |
| BCPC-30% | 4.53 ± 0.02 Ab | 5.31 ± 0.02 Bb | 5.23 ± 0.07 Cb | 4.82 ± 0.04 Db | 3.79 ± 0.04 Eb | 5.04 ± 0.03 Fb | 3.28 ± 0.03 Gb |
| Sample * | Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| n (-) | |||||||
| BCPC-20% | 0.60 ± 0.01 Aa | 0.95 ± 0.00 Ba | 0.91 ± 0.01 Ca | 0.73 ± 0.04 Da | 0.86 ± 0.01 Ea | 0.61 ± 0.03 Fa | 0.74 ± 0.00 Da |
| BCPC-30% | 0.58 ± 0.01 Aa | 0.97 ± 0.02 Bb | 0.91 ± 0.00 Ca | 0.79 ± 0.00 Db | 0.83 ± 0.01 Eb | 0.60 ± 0.01 Fa | 0.65 ± 0.00 Gb |
| K (Pa·sn) | |||||||
| BCPC-20% | 1.00 ± 0.02 Aa | 3.33 ± 0.10 Ba | 3.89 ± 0.05 Ca | 4.02 ± 0.02 Da | 4.85 ± 0.00 Ea | 4.83 ± 0.02 Ea | 8.05 ± 0.04 Fa |
| BCPC-30% | 1.08 ± 0.00 Aa | 4.15 ± 0.09 Bb | 6.72 ± 0.04 Cb | 5.12 ± 0.02 Db | 4.54 ± 0.01 Eb | 4.19 ± 0.02 Bb | 4.21 ± 0.02 Bb |
| τy (Pa) | |||||||
| BCPC-20% | 5.68 ± 0.11 Aa | 3.03 ± 0.05 Ba | 3.14 ± 0.01 Ca | 3.74 ± 0.01 Da | 6.59 ± 0.03 Ea | 9.37 ± 0.01 Fa | 9.64 ± 0.04 Ga |
| BCPC-30% | 3.17 ± 0.09 Ab | 4.78 ± 0.01 Bb | 4.40 ± 0.05 Cb | 3.41 ± 0.03 Db | 2.79 ± 0.02 Eb | 3.01 ± 0.02 Fb | 9.00 ± 0.01 Gb |
| Viscosity (Pa·s) | |||||||
| BCPC-20% | 160,000 ± 0.20 Aa | 13650 ± 0.54 Ba | 92260 ± 0.21 Ca | 8782 ± 0.15 Da | 8043 ± 0.09 Ea | 2407 ± 0.10 Fa | 56.72 ± 0.12 Ga |
| BCPC-30% | 659,000 ± 1.23 Ab | 970700 ± 0.34 Bb | 2111000 ± 1.12 Cb | 7987100 ± 0.43 Db | 103100 ± 0.45 Eb | 109.70 ± 0.21 Fb | 64.97 ± 0.52 Gb |
| yc (%) | |||||||
| BCPC-20% | 60.26 ± 0.20 Aa | 16.68 ± 0.05 Ba | 22.71 ± 0.03 Ca | 28.64 ± 0.09 Da | 76.05 ± 0.12 Ea | 63.08 ± 0.02 Fa | 39.43 ± 0.11 Ga |
| BCPC-30% | 18.85 ± 0.14 Ab | 24.16 ± 0.10 Bb | 16.06 ± 0.08 Cb | 17.67 ± 0.02 Db | 64.31 ± 0.05 Eb | 107.75 ± 0.15 Fb | 21.91 ± 0.08 Gb |
| Sample * | Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| Hardness (N) | |||||||
| BCPC-20% | 0.04 ± 0.00 Aa | 0.03 ± 0.00 Aa | 0.09 ± 0.00 Ba | 0.15 ± 0.02 Ca | 0.10 ± 0.00 Ba | 0.27 ± 0.01 Da | 0.36 ± 0.00 Ea |
| BCPC-30% | 0.04 ± 0.00 Aa | 0.05 ± 0.01 Ab | 0.19 ± 0.00 Bb | 0.25 ± 0.00 Cb | 0.27 ± 0.01 Db | 0.50 ± 0.01 Eb | 0.25 ± 0.02 CDb |
| Springiness (N) | |||||||
| BCPC-20% | 0.48 ± 0.04 Aa | 0.61 ± 0.02 Bb | 0.57 ± 0.02 Ca | 0.81 ± 0.02 Da | 0.69 ± 0.03 Ea | 0.79 ± 0.01 Da | 0.60 ± 0.02 Ba |
| BCPC-30% | 0.78 ± 0.02 Aa | 0.74 ± 0.02 Bb | 0.50 ± 0.06 Cb | 0.73 ± 0.01 Bb | 0.53 ± 0.03 Db | 0.80 ± 0.02 Aa | 0.59 ± 0.02 Ea |
| Gumminess (N) | |||||||
| BCPC-20% | 0.02 ± 0.00 Aa | 0.02 ± 0.01 Aa | 0.03 ± 0.01 ABa | 0.04 ± 0.01 Ca | 0.03 ± 0.01 BCPCa | 0.08 ± 0.00 Da | 0.10 ± 0.00 Ea |
| BCPC-30% | 0.24 ± 0.01 Ab | 0.18 ± 0.03 Bb | 0.20 ± 0.00 BCPCb | 0.26 ± 0.00 Db | 0.21 ± 0.00 Cb | 0.24 ± 0.01 Ab | 0.43 ± 0.01 Eb |
| Chewiness (N) | |||||||
| BCPC-20% | 0.01 ± 0.00 Aa | 0.01 ± 0.01 Aa | 0.02 ± 0.01 Aba | 0.03 ± 0.00 Ba | 0.02 ± 0.00 Aa | 0.07 ± 0.01 Ca | 0.06 ± 0.00 Ca |
| BCPC-30% | 0.20 ± 0.00 Ab | 0.13 ± 0.00 Bb | 0.10 ± 0.00 Cb | 0.19 ± 0.00 Ab | 0.14 ± 0.01 Db | 0.19 ± 0.02 Ab | 0.25 ± 0.06 Eb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Śmietana, N.; Paradowska, D.; Drozłowska, E. Black Cumin (Nigella sativa L.) Seed Press Cake as a Novel Material for the Development of New Non-Dairy Beverage Fermented with Kefir Grains. Microorganisms 2022, 10, 300. https://doi.org/10.3390/microorganisms10020300
Łopusiewicz Ł, Śmietana N, Paradowska D, Drozłowska E. Black Cumin (Nigella sativa L.) Seed Press Cake as a Novel Material for the Development of New Non-Dairy Beverage Fermented with Kefir Grains. Microorganisms. 2022; 10(2):300. https://doi.org/10.3390/microorganisms10020300
Chicago/Turabian StyleŁopusiewicz, Łukasz, Natalia Śmietana, Daria Paradowska, and Emilia Drozłowska. 2022. "Black Cumin (Nigella sativa L.) Seed Press Cake as a Novel Material for the Development of New Non-Dairy Beverage Fermented with Kefir Grains" Microorganisms 10, no. 2: 300. https://doi.org/10.3390/microorganisms10020300
APA StyleŁopusiewicz, Ł., Śmietana, N., Paradowska, D., & Drozłowska, E. (2022). Black Cumin (Nigella sativa L.) Seed Press Cake as a Novel Material for the Development of New Non-Dairy Beverage Fermented with Kefir Grains. Microorganisms, 10(2), 300. https://doi.org/10.3390/microorganisms10020300