Employment of L. paracasei K5 as a Novel Potentially Probiotic Freeze-Dried Starter for Feta-Type Cheese Production
Abstract
:1. Introduction
2. Materials and Methods
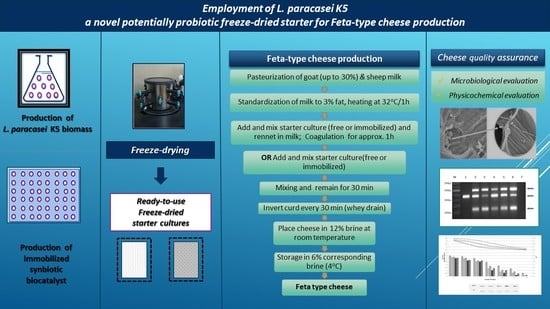
2.1. Starter Cultures for Cheese Production
2.2. Pilot-Scale Feta-Type Cheese Production
2.3. Physicochemical Analysis
2.4. Scanning Electron Microscopy—SEM
2.5. Microbiological Analyses
2.6. Molecular Detection of the Starter Culture in Cheese
2.7. Aromatic Volatiles Detection by SPME GC/MS
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Feta-Type Cheese
3.2. Microbiological Profile of Cheese Products
3.3. Survival of L. Paracasei K5 in Feta-Type Cheese during Storage
3.4. Impact of the Starter Culture on Aromatic Characteristics of Cheese
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Brown, L.; Caligiuri, S.P.B.; Brown, D.; Pierce, G.N. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gomes da Cruz, A.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Markets, R.A. Global Functional Food and Nutraceuticals Market (2016–2020). 2018. Available online: https://www.technavio.com/report/global-health-and-wellness-global-functional-food-and-nutraceuticals-market-2016-2020 (accessed on 18 December 2018).
- Gomes da Cruz, A.; Alonso Buriti, F.C.; Batista de Souza, C.H.; Fonseca Faria, J.A.; Isay Saad, S.M. Probiotic cheese: Health benefits, technological and stability aspects. Trends Food Sci. Technol. 2009, 20, 344–354. [Google Scholar] [CrossRef]
- Pisano, M.B.; Casula, M.; Corda, A.; Fadda, M.E.; Deplano, M.; Cosentino, S. In vitro probiotic characteristics of Lactobacillus strains isolated from Fiore Sardo cheese. Ital. J. Food Sci. 2008, 20, 505–516. [Google Scholar]
- Desai, A.R.; Shah, N.P.; Powell, I.B. Discrimination of Dairy Industry Isolates of the Lactobacillus casei Group. J. Dairy Sci. 2006, 89, 3345–3351. [Google Scholar] [CrossRef]
- Abriouel, H.; Casado Muñoz, M.d.C.; Lavilla Lerma, L.; Pérez Montoro, B.; Bockelmann, W.; Pichner, R.; Kabisch, J.; Cho, G.-S.; Franz, C.M.A.P.; Gálvez, A.; et al. New insights in antibiotic resistance of Lactobacillus species from fermented foods. Food Res. Int. 2015, 78, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Schoina, V.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin for functional myzithra cheese manufacture. LWT Food Sci. Technol. 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.I.; Bosnea, L.; Kanellaki, M.; Koutinas, A.A.; Castro, G.R. Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT Food Sci. Technol. 2017, 79, 616–624. [Google Scholar] [CrossRef]
- de Moraes, G.M.D.; dos Santos, K.M.O.; de Barcelos, S.C.; Lopes, S.A.; do Egito, A.S. Potentially probiotic goat cheese produced with autochthonous adjunct culture of Lactobacillus mucosae: Microbiological, physicochemical and sensory attributes. LWT 2018, 94, 57–63. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Bosnea, L.; Kanellaki, M.; Ganatsios, V.; Koutinas, A.A. Wheat bran as prebiotic cell immobilisation carrier for industrial functional Feta-type cheese making: Chemical, microbial and sensory evaluation. Biocatal. Agric. Biotechnol. 2018, 13, 75–83. [Google Scholar] [CrossRef]
- Moatsou, G.; Govaris, A. White brined cheeses: A diachronic exploitation of small ruminants milk in Greece. Small Rumin. Res. 2011, 101, 113–121. [Google Scholar] [CrossRef]
- Papadopoulou, O.S.; Argyri, A.A.; Varzakis, E.E.; Tassou, C.C.; Chorianopoulos, N.G. Greek functional Feta cheese: Enhancing quality and safety using a Lactobacillus plantarum strain with probiotic potential. Food Microbiol. 2018, 74, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, A.; Alexandraki, V.; Georgalaki, M.; Anastasiou, R.; Manolopoulou, E.; Tsakalidou, E.; Papadimitriou, K. Production of probiotic Feta cheese using Propionibacterium freudenreichii subsp. shermanii as adjunct. Int. Dairy J. 2017, 66, 135–139. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Karapetsas, A.; Kazakos, S.; Alexopoulos, A.; Mantzourani, I.; Chondrou, P.; Fournomiti, M.; Galanis, A.; Bezirtzoglou, E. Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from Feta-type cheese. Food Chem. 2017, 226, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Tsela, D.; Tiptiri-Kourpeti, A.; Anestopoulos, I.; Kotsianidis, I.; Bezirtzoglou, E.; Pappa, A.; Galanis, A. Lactobacillus paracasei K5 displays adhesion, anti-proliferative activity and apoptotic effects in human colon cancer cells. Benef. Microbes 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bosnea, L.A.; Kourkoutas, Y.; Albantaki, N.; Tzia, C.; Koutinas, A.A.; Kanellaki, M. Functionality of freeze-dried L. casei cells immobilized on wheat grains. LWT Food Sci. Technol. 2009, 42, 1696–1702. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Sypsas, V.; Kandylis, P.; Michelis, A.; Bekatorou, A.; Kourkoutas, Y.; Kordulis, C.; Lycourghiotis, A.; Banat, I.M.; Nigam, P.; et al. Nano-tubular cellulose for bioprocess technology development. PLoS ONE 2012, 7, e34350. [Google Scholar] [CrossRef]
- Engels, W.; Dusterhoft, E.-M.; Huppertz, T. Starter Cultures for Cheese Manufacture. In Reference Module in Food Science; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- De Assumpção Pereira-da-Silva, M.; Ferri, F.A. 1—Scanning Electron Microscopy. In Nanocharacterization Techniques; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 1–35. [Google Scholar]
- Terpou, A.; Nigam, P.S.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT Food Sci. Technol. 2018, 97, 109–116. [Google Scholar] [CrossRef]
- Tabla, R.; Gómez, A.; Simancas, A.; Rebollo, J.E.; Molina, F.; Roa, I. Enterobacteriaceae species during manufacturing and ripening of semi-hard and soft raw ewe’s milk cheese: Gas production capacity. Small Rumin. Res. 2016, 145, 123–129. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E.; Samelis, J. Microbiological and biochemical characteristics of Kashkaval cheese produced using pasteurised or raw milk. Int. Dairy J. 2019, 89, 60–67. [Google Scholar] [CrossRef]
- Ullah, N.; Wang, X.; Wu, J.; Guo, Y.; Ge, H.; Li, T.; Khan, S.; Li, Z.; Feng, X. Purification and primary characterization of a novel bacteriocin, LiN333, from Lactobacillus casei, an isolate from a Chinese fermented food. Lwt Food Sci. Technol. 2017, 84, 867–875. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Kandylis, P.; Sidira, M.; Koutinas, A.A.; Kourkoutas, Y. Free and immobilized Lactobacillus casei ATCC 393 on whey protein as starter cultures for probiotic Feta-type cheese production. J. Dairy Sci. 2014, 97, 4675–4685. [Google Scholar] [CrossRef]
- Yuvaşen, A.; Macit, E.; Dertli, E. Microbial species playing roles for the production of traditional Kasar cheese during pre-maturation period. LWT 2018, 91, 406–413. [Google Scholar] [CrossRef]
- Moubasher, A.A.H.; Abdel-Sater, M.A.; Soliman, Z.S.M. Yeasts and filamentous fungi associated with some dairy products in Egypt. J. Mycol. Med. 2018, 28, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, B.C. The interaction between yeasts and bacteria in dairy environments. Int. J. Food Microbiol. 2001, 69, 37–44. [Google Scholar] [CrossRef]
- Voulgari, K.; Hatzikamari, M.; Delepoglou, A.; Georgakopoulos, P.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Antifungal activity of non-starter lactic acid bacteria isolates from dairy products. Food Control 2010, 21, 136–142. [Google Scholar] [CrossRef]
- Terpou, A.; Bosnea, L.; Kanellaki, M.; Plessas, S.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A. Growth Capacity of a Novel Potential Probiotic Lactobacillus paracasei K5 Strain Incorporated in Industrial White Brined Cheese as an Adjunct Culture. J. Food Sci. 2018, 83, 723–731. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.-I.; Bekatorou, A.; Dimitrellou, D.; Ganatsios, V.; Barouni, E.; Koutinas, A.A.; Kanellaki, M. Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food Bioprod. Process. 2017, 101, 184–192. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 2003, 82, 133–141. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Shori, A.B. The potential applications of probiotics on dairy and non-dairy foods focusing on viability during storage. Biocatal. Agric. Biotechnol. 2015, 4, 423–431. [Google Scholar] [CrossRef]
- Domingos, L.D.; de Souza, H.A.L.; Mariutti, L.R.B.; de Toledo Benassi, M.; Bragagnolo, N.; Viotto, W.H. Fat reduction and whey protein concentrate addition alter the concentration of volatile compounds during Prato cheese ripening. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Bezerra, T.K.A.; Arcanjo, N.M.d.O.; Araújo, A.R.R.d.; Queiroz, A.L.M.d.; Oliveira, M.E.G.d.; Gomes, A.M.P.; Madruga, M.S. Volatile profile in goat coalho cheese supplemented with probiotic lactic acid bacteria. LWT Food Sci. Technol. 2017, 76, 209–215. [Google Scholar] [CrossRef]
- Sádecká, J.; Šaková, N.; Pangallo, D.; Koreňová, J.; Kolek, E.; Puškárová, A.; Bučková, M.; Valík, L.; Kuchta, T. Microbial diversity and volatile odour-active compounds of barrelled ewes’ cheese as an intermediate product that determines the quality of winter bryndza cheese. LWT Food Sci. Technol. 2016, 70, 237–244. [Google Scholar] [CrossRef]
- Salum, P.; Govce, G.; Kendirci, P.; Bas, D.; Erbay, Z. Composition, proteolysis, lipolysis, volatile compound profile and sensory characteristics of ripened white cheeses manufactured in different geographical regions of Turkey. Int. Dairy J. 2018, 87, 26–36. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Wu, T.; Min, W.; Yang, Z. Exopolysaccharide producing Lactobacillus plantarum SKT109 as adjunct culture in Cheddar cheese production. LWT 2018, 97, 419–426. [Google Scholar] [CrossRef]
- Izco, J.M.; Torre, P. Characterisation of volatile flavour compounds in Roncal cheese extracted by the ‘purge and trap’ method and analysed by GC–MS. Food Chem. 2000, 70, 409–417. [Google Scholar] [CrossRef]
- Castillo, I.; Calvo, M.V.; Alonso, L.; Juárez, M.; Fontecha, J. Changes in lipolysis and volatile fraction of a goat cheese manufactured employing a hygienized rennet paste and a defined strain starter. Food Chem. 2007, 100, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Andiç, S.; Tunçtürk, Y.; Boran, G. Chapter 28—Changes in Volatile Compounds of Cheese. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 231–239. [Google Scholar]
- Alewijn, M.; Smit, B.A.; Sliwinski, E.L.; Wouters, J.T.M. The formation mechanism of lactones in Gouda cheese. Int. Dairy J. 2007, 17, 59–66. [Google Scholar] [CrossRef]





| Time (days) | C1 | C2 | C3 | C4 | C5 | |
|---|---|---|---|---|---|---|
| TBC (log cfu/g) | 1 | 7.1 ± 0.1 a | 7.7 ± 0.2 b | 8 ± 0.1 bc | 8.57 ± 0.06 d | 8.27 ± 0.25 cd |
| 5 | 8.4 ± 0.1 a | 7.67 ± 0.06 b | 8.3 ± 0.1 a | 8.2 ± 0.2 a | 8.2 ± 0.2 a | |
| 10 | 8.33 ± 0.06 a | 8.23 ± 0.06 a | 7.9 ± 0.1 b | 8.03 ± 0.06 b | 8.53 ± 0.06 c | |
| 15 | 8.83 ± 0.06 a | 7.8 ± 0.1 b | 7.47 ± 0.06 c | 8.1 ± 0.1 d | 8.23 ± 0.06 d | |
| 30 | 8.37 ± 0.21 a | 8.13 ± 0.15 ab | 7.87 ± 0.06 bc | 7.67 ± 0.06 c | 7.73 ± 0.06 c | |
| 45 | 8.1 ± 0.1 a | 7.9 ± 0.1 ab | 7.7 ± 0.1 bc | 7.6 ± 0 bc | 7.5 ± 0.2 c | |
| 55 | 8 ± 0.1 a | 7.5 ± 0.2 b | 7.37 ± 0.06 bd | 6.33 ± 0.06 c | 7.17 ± 0.06 d | |
| 60 | 7.4 ± 0.2 a | 6.87 ± 0.06 b | 6.77 ± 0.06 b | 5.6 ± 0.1 c | 6.67 ± 0.06 b | |
| 75 | 7.03 ± 0.06 a | 6.5 ± 0.17 b | 6.67 ± 0.12 b | 5.23 ± 0.15 c | 6.1 ± 0.1 d | |
| 90 | 6.67 ± 0.06 a | 6.7 ± 0.1 a | 6.13 ± 0.06 b | 4.63 ± 0.31 c | 5.8 ± 0.1 b | |
| Count of Lactobacillus genus bacteria (log cfu/g) | 1 | 5.23 ± 0.15 a | 8.1 ± 0.1 b | 7.63 ± 0.06 c | 8.5 ± 0.1 d | 8.2 ± 0.1 b |
| 5 | 5.9 ± 0.1 a | 8.3 ± 0.1 b | 8.13 ± 0.15 b | 8.7 ± 0.1 c | 8.63 ± 0.06 c | |
| 10 | 6.1 ± 0.1 a | 8.6 ± 0.1 bc | 8.3 ± 0.1 b | 8.8 ± 0.2 c | 8.5 ± 0.1 bc | |
| 15 | 6.2 ± 0.1 a | 8.5 ± 0.1 bc | 8.4 ± 0.1 b | 8.6 ± 0.1 bc | 8.7 ± 0.1 c | |
| 30 | 5.7 ± 0.3 a | 8.6 ± 0.2 b | 7.9 ± 0.1 c | 8.6 ± 0.1 b | 8.5 ± 0.1 b | |
| 45 | 5.4 ± 0.1 a | 8.7 ± 0.1 b | 8.03 ± 0.06 c | 8.37 ± 0.06 d | 8.3 ± 0.1 d | |
| 55 | 5.47 ± 0.06 a | 8.17 ± 0.06 bc | 7.7 ± 0.1 d | 8.33 ± 0.06 c | 8.03 ± 0.06 b | |
| 60 | 5.2 ± 0.1 a | 7.7 ± 0.1 b | 7.5 ± 0.1 b | 8.5 ± 0.1 c | 7.7 ± 0.1 b | |
| 75 | 4.67 ± 0.15 a | 7.4 ± 0.1 b | 7.2 ± 0.2 b | 8.03 ± 0.06 c | 7.5 ± 0.1 b | |
| 90 | 4.2 ± 0.2 a | 7.1 ± 0.1 b | 6.7 ± 0.1 c | 7.7 ± 0.1 d | 7.1 ± 0.2 b | |
| Count of Lactococcus genus bacteria (log cfu/g) | 1 | 7.7 ± 0.2 a | 7.3 ± 0.2 b | 7.6 ± 0.1 ab | 6.7 ± 0.1 c | 7.7 ± 0.1 a |
| 5 | 8.2 ± 0.1 a | 7.7 ± 0.1 b | 7.4 ± 0.2 b | 6.4 ± 0.1 c | 7.4 ± 0.1 b | |
| 10 | 7.9 ± 0.1 a | 7.5 ± 0.1 b | 6.73 ± 0.06 c | 6.2 ± 0.1 d | 7.6 ± 0.1 b | |
| 15 | 7.6 ± 0.2 a | 7.8 ± 0.1 a | 6.3 ± 0.1 b | 5.9 ± 0.1 c | 7.2 ± 0.1 d | |
| 30 | 7.5 ± 0.1 a | 6.6 ± 0.1 b | 6.4 ± 0.1 b | 5.9 ± 0.1 c | 6.6 ± 0.1 b | |
| 45 | 7.7 ± 0.1 a | 6.8 ± 0.1 b | 6.4 ± 0.1 c | 4.6 ± 0.2 d | 5.27 ± 0.06 e | |
| 55 | 7.4 ± 0.1 a | 6.1 ± 0.1 b | 4.97 ± 0.15 c | 4.1 ± 0.1 c | 4.73 ± 0.06 d | |
| 60 | 6.93 ± 0.06 a | 5.8 ± 0.2 b | 4.7 ± 0.1 c | 3.7 ± 0.1 d | 4.1 ± 0.1 e | |
| 75 | 6.5 ± 0.1 a | 5.5 ± 0.1 b | 4.1 ± 0.1 c | 3.2 ± 0.2 d | 3.7 ± 0.1 e | |
| 90 | 6.13 ± 0.06 a | 5.1 ± 0.1 b | 4 ± 0.2 c | 3 ± 0.1 d | 3.33 ± 0.06 e | |
| Count of Enterobacteriaceae family bacteria (log cfu/g) | 1 | 2.9 ± 0.1 a | 2.4 ± 0.2 b | 2.2 ± 0.2 bc | 1.9 ± 0.1 c | 2.4 ± 0.1 b |
| 5 | 4.8 ± 0.1 a | 2.2 ± 0.1 b | 2.27 ± 0.15 b | 2.3 ± 0.1 bc | 2.6 ± 0.1 c | |
| 10 | 5.3 ± 0.2 a | 3.6 ± 0.1 b | 2.7 ± 0.1 c | 1.6 ± 0.2 d | 2.9 ± 0.1 c | |
| 15 | 4.4 ± 0.2 a | 3.1 ± 0.1 b | 2 ± 0.2 c | 1.8 ± 0.2 c | 2.2 ± 0.4 c | |
| 30 | 4.1 ± 0.1 a | 2.7 ± 0.1 b | 2.27 ± 0.25 c | 1.03 ± 0.06 d | 1.6 ± 0.1 e | |
| 45 | 3.6 ± 0.1 a | 2.1 ± 0.1 b | 1.7 ± 0.1 c | nd | 1.07 ± 0.06 d | |
| 55 | 3.3 ± 0.1 a | 1.6 ± 0.1 b | 1.6 ± 0.1 b | nd | nd | |
| 60 | 2.8 ± 0.2 a | 1.3 ± 0.1 b | 1.3 ± 0.2 b | nd | nd | |
| 75 | 2.6 ± 0.1 | nd | nd | nd | ||
| 90 | 2.4 ± 0.1 | nd | nd | nd | nd | |
| Count of Yeasts & Fungi (log cfu/g) | 1 | 6.4 ± 0.1 a | 5.8 ± 0.2 b | 6.6 ± 0.1 a | 6.7 ± 0.1 a | 6.6 ± 0.1 a |
| 5 | 5.7 ± 0.1 a | 5.1 ± 0.1 b | 6.2 ± 0.1 c | 6.2 ± 0.1 c | 7.2 ± 0.2 d | |
| 10 | 6.1 ± 0.1 a | 5.3 ± 0.2 b | 5.7 ± 0.1 c | 6 ± 0.1 ac | 7 ± 0.1 d | |
| 15 | 5.8 ± 0.1 a | 5.2 ± 0.1 b | 6.1 ± 0.1 c | 6.03 ± 0.06 ac | 6.8 ± 0.1 d | |
| 30 | 5.6 ± 0.1 a | 4.8 ± 0.1 b | 5.4 ± 0.1 a | 5.7 ± 0.1 a | 6.2 ± 0.2 c | |
| 45 | 5.9 ± 0.2 a | 4.1 ± 0.1 b | 5.2 ± 0.1 c | 5.2 ± 0.1 c | 5.7 ± 0.1 a | |
| 55 | 6.2 ± 0.1 a | 4.1 ± 0.1 b | 4.9 ± 0.1 c | 5.03 ± 0.06 c | 5.1 ± 0.1 c | |
| 60 | 5.9 ± 0.1 a | 3.8 ± 0.2 b | 4.4 ± 0.2 c | 4.3 ± 0.1 c | 4.9 ± 0.1 d | |
| 75 | 5.4 ± 0.2 a | 3.4 ± 0.2 b | 4.2 ± 0.2 c | 3.6 ± 0.1 b | 4.6 ± 0.1 c | |
| 90 | 4.8 ± 0.1 a | 3.1 ± 0.1 b | 3.7 ± 0.1 b | 3.2 ± 0.1 c | 4.4 ± 0.1 d | |
| Count of Staphylococcus genus bacteria (log cfu/g) | 1 | 1.1 ± 0.1 a | 1.4 ± 0.2 b | 1.0 ± 0.0 a | 1.1 ± 0.1 ab | 1.2 ± 0.1 ab |
| 5 | 1.7 ± 0.1 ab | 1.3 ± 0.1 c | 1.3 ± 0.2 c | 1.4 ± 0.1 ac | 1.9 ± 0.1 b | |
| 10 | 1.4 ± 0.1 a | 1.1 ± 0.1 b | 1.4 ± 0.1 a | 1.1 ± 0.1 b | 1.7 ± 0.1 c | |
| 15 | 1.27 ± 0.25 ab | 1.0 ± 0.0 a | 1.6 ± 0.1 b | 1.0 ± 0.0 a | 2.1 ± 0.1 c | |
| 30 | 1.1 ± 0.1 a | nd | 1.2 ± 0.1 ac | 1.03 ± 0.06 a | 1.4 ± 0.1 c | |
| 45 | 1.0 ± 0.0 a | nd | 1.0 ± 0.0 a | nd | 1.1 ± 0.1 a | |
| 55 | 1.1 ± 0.1 a | nd | nd | nd | 1.0 ± 0.0 a | |
| 60 | 1.0 ± 0.0 | nd | nd | nd | nd | |
| 75 | 1.0 ± 0.0 | nd | nd | nd | nd | |
| 90 | 1.0 ± 0.0 | nd | nd | nd | nd |
| Compound | Identification Methods α | KI β | C1 * | C2 * | C3 * | C4 * | C5 * |
|---|---|---|---|---|---|---|---|
| Esters | |||||||
| Ethyl butanoate | MS, KI | 1041 | 1.07 ± 0.03 a | 0.17 ± 0.02 b | 0.26 ± 0.02 c | 0.10 ± 0.03 b | 0.19 ± 0.05 bc |
| Ethyl hexanoate | MS, KI | 1251 | 0.00 ± 0.00 a | 0.14 ± 0.03 b | 0.11 ± 0.02 b | 2.60 ± 0.04 c | 2.16 ± 0.04 d |
| Hexyl acetate | MS, KI | 1284 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.44 ± 0.58 a | 0.08 ± 0.02 a |
| Methyl octanoate | MS, KI | 1380 | 0.00 ± 0.00 a | 1.14 ± 0.02 b | 1.36 ± 0.02 c | 1.26 ± 0.03 d | 1.04 ± 0.04 e |
| Ethyl octanoate | MS, KI | 1421 | 1.06 ± 0.01 a | 5.24 ± 0.05 b | 6.00 ± 0.05 c | 6.72 ± 0.02 d | 6.45 ± 0.04 e |
| Ethyl decanoate | MS, KI | 1634 | 8.84 ± 0.05 a | 16.70 ± 0.60 b | 12.54 ± 0.05 c | 26.15 ± 0.05 d | 20.25 ± 0.05 e |
| 2-Phenylethyl acetate | MS, KI | 1830 | 1.35 ± 0.03 a | 3.03 ± 0.02 b | 4.15 ± 0.05 c | 3.07 ± 0.07 b | 2.23 ± 0.04 d |
| Ethyl dodecanoate | MS, KI | 1848 | 7.15 ± 0.06 a | 11.15 ± 0.09 b | 10.48 ± 0.03 c | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| Free Fatty acids | |||||||
| Butanoic acid (C4:0) | MS, KI | 1642 | 76.16 ± 0.06 a | 87.14 ± 0.11 b | 79.08 ± 1.85 c | 68.74 ± 0.27 d | 87.46 ± 0.49 b |
| Hexanoic acid (C6:0) | MS, KI | 1851 | 121.33 ± 11.50 a | 385.73 ± 5.56 b | 273.63 ± 3.26 c | 391.97 ± 3.78 b | 385.83 ± 2.44 b |
| Octanoic acid (C8:0) | MS, KI | 2064 | 70.73 ± 5.40 a | 187.10 ± 6.16 b | 167.33 ± 3.58 c | 205.00 ± 6.52 d | 192.40 ± 3.12 bd |
| Decanoic acid (C10:0) | MS, KI | 2336 | 189.13 ± 3.65 a | 267.70 ± 2.72 b | 243.23 ± 1.36 c | 314.00 ± 4.33 d | 239.20 ± 8.86 c |
| Dodecanoic acid (C12:0) | MS, KI | 2485 | 67.57 ± 5.27 a | 79.54 ± 2.75 b | 78.93 ± 5.42 ab | 88.15 ± 5.51 b | 87.69 ± 2.01 b |
| Alcohols | |||||||
| ethanol | MS, KI | 932 | >10.000 | " | " | " | " |
| 3-Methyl-1-butanol | MS, KI | 1216 | 0.00 ± 0.00 a | 4.82 ± 0.30 b | 4.22 ± 0.06 c | 6.18 ± 0.03 d | 5.25 ± 0.10 e |
| 1-Hexanol | MS, KI | 1363 | 1.01 ± 0.04 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 1-octen-3-ol | MS, KI | 1457 | 0.00 ± 0.00 a | 0.18 ± 0.07 a | 0.50 ± 0.57 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 1-Octanol | MS, KI | 1555 | 0.00 ± 0.00 a | 1.25 ± 0.05 b | 1.11 ± 0.03 b | 3.17 ± 0.10 c | 3.11 ± 0.19 c |
| 2,3 butanediol | MS, KI | 1569 | 1.04 ± 0.04 a | 55.37 ± 93.21 a | 1.67 ± 0.05 a | 4.45 ± 2.92 a | 4.07 ± 0.05 a |
| Phenyl ethanol | MS, KI | 1932 | 18.40 ± 0.54 a | 7.16 ± 0.53 b | 10.03 ± 0.14 c | 8.07 ± 0.05 b | 12.29 ± 0.48 d |
| Carbonyl compou0s | |||||||
| propanal | MS | <800 | 0.11 ± 0.02 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| hexanal | MS, KI | 1088 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.12 ± 0.06 b | 0.00 ± 0.00 a |
| octanal | MS, KI | 1301 | 0.00 ± 0.00 a | 0.14 ± 0.01 b | 0.26 ± 0.05 c | 0.18 ± 0.01 b | 0.73 ± 0.04 d |
| 3-hydroxy,2-butanone | MS, KI | 1310 | 0.00 ± 0.00 a | 2.27 ± 0.06 b | 2.67 ± 0.04 c | 1.25 ± 0.04 d | 3.13 ± 0.09 e |
| Nonanal | MS, KI | 1395 | 1.75 ± 0.03 a | 6.15 ± 0.12 b | 6.77 ± 0.24 b | 8.56 ± 0.57 c | 7.57 ± 0.02 d |
| Benzaldehyde | MS, KI | 1528 | 2.15 ± 0.12 a | 0.81 ± 0.01 b | 1.22 ± 0.06 c | 0.15 ± 0.04 d | 2.14 ± 0.07 a |
| Lactones | |||||||
| δ-decalactone | MS, KI | 2209 | 1.05 ± 0.04 a | 12.16 ± 0.06 b | 12.84 ± 0.61 bc | 15.46 ± 1.53 c | 11.91 ± 1.88 b |
| γ- dodecalactone | MS, KI | 2388 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 3.15 ± 0.04 b | 2.82 ± 0.57 b |
| δ-dodecalactone | MS, KI | 2437 | 3.05 ± 0.05 a | 10.14 ± 0.12 b | 8.07 ± 0.65 c | 10.73 ± 1.17 b | 10.12 ± 0.06 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpou, A.; Mantzourani, I.; Galanis, A.; Kanellaki, M.; Bezirtzoglou, E.; Bekatorou, A.; Koutinas, A.A.; Plessas, S. Employment of L. paracasei K5 as a Novel Potentially Probiotic Freeze-Dried Starter for Feta-Type Cheese Production. Microorganisms 2019, 7, 3. https://doi.org/10.3390/microorganisms7010003
Terpou A, Mantzourani I, Galanis A, Kanellaki M, Bezirtzoglou E, Bekatorou A, Koutinas AA, Plessas S. Employment of L. paracasei K5 as a Novel Potentially Probiotic Freeze-Dried Starter for Feta-Type Cheese Production. Microorganisms. 2019; 7(1):3. https://doi.org/10.3390/microorganisms7010003
Chicago/Turabian StyleTerpou, Antonia, Ioanna Mantzourani, Alex Galanis, Maria Kanellaki, Eugenia Bezirtzoglou, Argyro Bekatorou, Athanasios A. Koutinas, and Stavros Plessas. 2019. "Employment of L. paracasei K5 as a Novel Potentially Probiotic Freeze-Dried Starter for Feta-Type Cheese Production" Microorganisms 7, no. 1: 3. https://doi.org/10.3390/microorganisms7010003
APA StyleTerpou, A., Mantzourani, I., Galanis, A., Kanellaki, M., Bezirtzoglou, E., Bekatorou, A., Koutinas, A. A., & Plessas, S. (2019). Employment of L. paracasei K5 as a Novel Potentially Probiotic Freeze-Dried Starter for Feta-Type Cheese Production. Microorganisms, 7(1), 3. https://doi.org/10.3390/microorganisms7010003