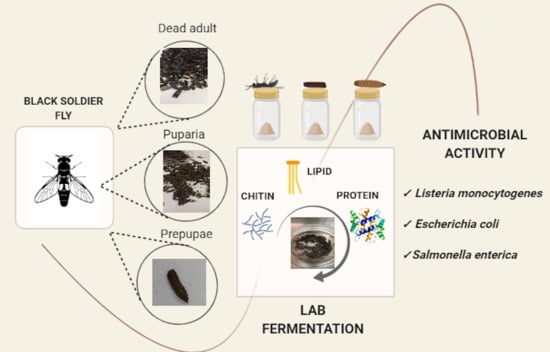
Antimicrobial Biomasses from Lactic Acid Fermentation of Black Soldier Fly Prepupae and Related By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Insect Waste
2.2. Bacterial Strains
2.3. Fermentation
2.4. Proximate Composition
2.5. Total Amino Acid Profile
Tryptophan Determination by UPLC/ESI-MS after Alkaline Hydrolysis
2.6. Determination of Chitin
2.7. Determination of Fatty Acids Profile by GC-MS
2.8. Microbial Challenge Test
2.9. Statistical Analysis
3. Results
3.1. Fermentation
3.2. Black Soldier Fly Composition
3.3. Variations of Fatty Acid Profile after Fermentation
3.4. Variations of Total Amino Acid Profile after Fermentation
3.5. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Edible Insects. Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Roma, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Garofalo, C.; Milanović, V.; Cardinali, F.; Aquilanti, L.; Clementi, F.; Osimani, A. Current knowledge on the microbiota of edible insects intended for human consumption: A state-of-the-art review. Food Res. Int. 2019, 125. [Google Scholar] [CrossRef]
- Jansson, A.; Berggren, A. Insects as Food—Something for the Future? Lotta, R., Pernilla, J., Eds.; Future Agriculture, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2015; ISBN 978-91-576-9335-8. [Google Scholar]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S.; et al. Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varelas, V. Food wastes as a potential new source for edible insect mass production for food and feed: A review. Fermentation 2019, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State of the art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014. [Google Scholar] [CrossRef]
- EU Regulation (EU). 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliam; EU Regulation: Maastricht, The Netherlands, 2017; Volume 60, pp. 1–120. [Google Scholar]
- De Smet, J.; Wynants, E.; Cos, P.; Van Campenhout, L. Microbial Community Dynamics during Rearing of Black Soldier Fly Larvae (Hermetia illucens) and Impact on Exploitation Potential. Appl. Environ. Microbiol. 2018, 84, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Barbi, S.; Macavei, L.I.; Fuso, A.; Luparelli, A.V.; Caligiani, A.; Ferrari, A.M.; Maistrello, L.; Montorsi, M. Valorization of seasonal agri-food leftovers through insects. Sci. Total Environ. 2020, 709. [Google Scholar] [CrossRef]
- Bortolini, S.; Macavei, L.I.; Hadj Saadoun, J.; Foca, G.; Ulrici, A.; Bernini, F.; Malferrari, D.; Setti, L.; Ronga, D.; Maistrello, L. Hermetia illucens (L.) larvae as chicken manure management tool for circular economy. J. Clean. Prod. 2020, 262, 121289. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of Vegetable and Fruit Substrates as Potential Rearing Media for Hermetia illucens (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Montevecchi, G.; Zanasi, L.; Bortolini, S.; Macavei, L.I.; Masino, F.; Maistrello, L.; Antonelli, A. Lipid profile and growth of black soldier flies (Hermetia illucens, Stratiomyidae) reared on by-products from different food chains. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef] [PubMed]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of Life Cycle Assessment to process using Hermetia illucens. J. Clean. Prod. 2017. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Meticulous Research Black Soldier Fly Market Worth $2.57 Billion by 2030. Available online: https://www.globenewswire.com/news-release/2020/01/15/1970839/0/en/Black-Soldier-Fly-Market-Worth-2-57-Billion-by-2030-Exclusive-Report-by-Meticulous-Research.html (accessed on 23 June 2020).
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From byproduct to resource: Fermented apple pomace as beer flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, A.; Diaz, A.B.; Caro, I.; Bernini, V.; Galaverna, G.; Lazzi, C.; Blandino, A. Orange peels: From by-product to resource through lactic acid fermentation. J. Sci. Food Agric. 2019, 99, 6761–6767. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable by-product lacto-fermentation as a new source of antimicrobial compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef] [Green Version]
- Borremans, A.; Lenaerts, S.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Marination and fermentation of yellow mealworm larvae (Tenebrio molitor). Food Control 2018. [Google Scholar] [CrossRef]
- Hogsette, J.A. New Diets for Production of House Flies and Stable Flies (Diptera: Muscidae) in the Laboratory. J. Econ. Entomol. 1992, 85, 2291–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing Methods for the Black Soldier Fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benassi, M.; Giovanni, A.; Benassi, G.; Maistrello, L.; Macavei, L.I.; Bortolini, S.; Hadj Saadoun, J. Dispositivo per la Deposizione di Uova di Ditteri Stratiomiidi e un Apparato per L’allevamento di Ditteri Stratiomiidi Comprendente Detto Dispositivo. Italy Patent 102018000003261, 3 May 2018. [Google Scholar]
- Association of Official Analytical Chemist, and A. of O.A.C. (US) Official Methods of Analysis Association of Official Analytical Chemist 1950. Volume 9. Available online: https://www.aoac.org/ (accessed on 22 January 2020).
- Leni, G.; Caligiani, A.; Sforza, S. Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: A metabolomics and proteomic insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- D’Hondt, E.; Soetemans, L.; Bastiaens, L.; Maesen, M.; Jespers, V.; Van den Bosch, B.; Voorspoels, S.; Elst, K. Simplified determination of the content and average degree of acetylation of chitin in crude black soldier fly larvae samples. Carbohydr. Res. 2020, 488. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Choi, W.H.; Yun, J.H.; Chu, J.P.; Chu, K.B. Antibacterial effect of extracts of Hermetia illucens (diptera: Stratiomyidae) larvae against gram-negative bacteria. Entomol. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- Choi, W.H.; Jiang, M. Evaluation of antibacterial activity of hexanedioic acid isolated from Hermetia illucens larvae. J. Appl. Biomed. 2014, 12, 179–189. [Google Scholar] [CrossRef]
- Liu, Q.; Tomberlin, J.K.; Brady, J.A.; Sanford, M.R.; Yu, Z. Black Soldier Fly (Diptera: Stratiomyidae) Larvae Reduce Escherichia coli in Dairy Manure. Environ. Entomol. 2008, 37, 1525–1530. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Callegari, M.; Jucker, C.; Fusi, M.; Leonardi, M.G.; Daffonchio, D.; Borin, S.; Savoldelli, S.; Crotti, E. Hydrolytic Profile of the Culturable Gut Bacterial Community Associated With Hermetia illucens. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier (Hermetia illucens L.) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Anaya, J.; Hernández-Santoyo, A. Production of bioactive conjugated linoleic acid by the multifunctional enolase from Lactobacillus plantarum. Int. J. Biol. Macromol. 2016, 91, 524–535. [Google Scholar] [CrossRef]
- Bao, Z.; Xiong, J.; Lin, W.; Ye, J. Profiles of free fatty acids, free amino acids, and volatile compounds of milk bases fermented by Lactobacillus casei GBHM-21 with different fat levels. CyTA-J. Food 2016, 14, 10–17. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Z.; Zou, Y.; He, R.; Ju, X.; Yuan, J. Effect of static-state fermentation on volatile composition in rapeseed meal. J. Sci. Food Agric. 2020, 100, 2145–2152. [Google Scholar] [CrossRef]
- Wang, D.H.; Yang, Y.; Wang, Z.; Lawrence, P.; Worobo, R.W.; Brenna, J.T. High levels of branched chain fatty acids in nātto and other Asian fermented foods. Food Chem. 2019, 286, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Kieronczyk, A.; Skeie, S.; Olsen, K.; Langsrud, T. Metabolism of amino acids by resting cells of non-starter lactobacilli in relation to flavour development in cheese. Proc. Int. Dairy J. 2001, 11, 217–224. [Google Scholar] [CrossRef]
- Liu, J.Z.; Weng, L.P.; Zhang, Q.L.; Xu, H.; Ji, L.N. A mathematical model for gluconic acid fermentation by Aspergillus niger. Biochem. Eng. J. 2003, 14, 137–141. [Google Scholar] [CrossRef]
- Tammam, J.D.; Williams, A.G.; Noble, J.; Lloyd, D. Amino acid fermentation in non-starter Lactobacillus spp. isolated from Cheddar cheese. Lett. Appl. Microbiol. 2000, 30, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.A.; Verstegen, M.W.A.; Tamminga, S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 2001, 14, 207–228. [Google Scholar] [CrossRef]
- Andersen, S.O. Cuticular Sclerotization and Tanning. In Insect Molecular Biology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 167–192. ISBN 9780123847478. [Google Scholar]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Rosmawati, A.; Rumhayati, B.; Srihardyastutie, A. Biorecovery of chitin from shrimp shell waste (Litopenaeus vanamme) using fermentation and co-fermentation of L. plantarum and B. thuringiensis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.P.; Jia, S.R.; Chu, L.Q.; Xie, Y.Y.; Li, Z.X.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef]
- Haghighi, H.; Leugoue, S.K.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Fava, P.; Pulvirenti, A. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020, 100, 105419. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26. [Google Scholar] [CrossRef]
- Leisner, J.J.; Vogensen, F.K.; Kollmann, J.; Aideh, B.; Vandamme, P.; Vancanneyt, M.; Ingmer, H. α-Chitinase activity among lactic acid bacteria. Syst. Appl. Microbiol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; González-Tejedo, C.; Ruas-Madiedo, P.; Urdaci, M.C.; Margolles, A. Lactobacillus plantarum extracellular chitin-binding protein and its role in the interaction between chitin, caco-2 cells, and mucin. Appl. Environ. Microbiol. 2011, 77, 1123–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| L. plantarum 285 | L. rhamnosus 1473 | |||||
|---|---|---|---|---|---|---|
| T0 | T72 | △ (T72–T0) | T0 | T72 | △ (T72–T0) | |
| Prepupae | 5.61 ± 0.68 | 7.80 ± 0.57 | 2.19 | 4.81 ± 1.30 | 2.64 ± 0.36 | −2.17 |
| Puparia | 7.19 ± 0.28 | 9.36 ± 0.10 | 2.17 | 7.15 ± 0.64 | 9.11 ± 0.29 | 1.96 |
| Dead Adults | 6.65 ± 0.43 | 8.84 ± 0.48 | 2.19 | 4.29 ± 0.81 | 7.33 ± 0.49 | 3.04 |
| Composition (%) * | L. plantarum 285 | L. rhamnosus 1473 | ||||
|---|---|---|---|---|---|---|
| Prepupae | Puparia | Dead Adults | Prepupae | Puparia | Dead Adults | |
| Moisture (Oven, 105 °C 24 h) | 67 ± 0.3 | 68.4 ± 2.00 | 76.4 ± 0.40 | 68.07 ± 0.02 | 74.0 ± 1.70 | 76.51 ± 0.05 |
| Lipid (Soxhlet) | 5.3 ± 0.5 | 1.7 ± 0.60 | 3.85 ± 0.01 | 3.30 ± 0.90 | 2.28 ± 0.03 | 4.30 ± 0.10 |
| Proteins, from total AA (UPLC/ESI-MS) | 11 ± 1.00 | 7 ± 1.00 | 9.34 ± 0.00 | 11.15 ± 0.06 | 6.4 ± 0.30 | 9.60 ± 0.70 |
| Chitin (UPLC/ESI-MS Determination of Glucosamine) | 1.8 ± 0.20 | 5.2 ± 0.60 | 1.29 ± 0.09 | 1.70 ± 0.10 | 3.9 ± 0.30 | 1.4 ± 0.10 |
| Ash (Oven 550 °C 5 h + 5 h) | 2.07 ± 0.06 | 5.75 ± 0.02 | 1.00 ± 0.10 | 2.00 ± 0.10 | 4.7 ± 1.10 | 0.82 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadj Saadoun, J.; Luparelli, A.V.; Caligiani, A.; Macavei, L.I.; Maistrello, L.; Neviani, E.; Galaverna, G.; Sforza, S.; Lazzi, C. Antimicrobial Biomasses from Lactic Acid Fermentation of Black Soldier Fly Prepupae and Related By-Products. Microorganisms 2020, 8, 1785. https://doi.org/10.3390/microorganisms8111785
Hadj Saadoun J, Luparelli AV, Caligiani A, Macavei LI, Maistrello L, Neviani E, Galaverna G, Sforza S, Lazzi C. Antimicrobial Biomasses from Lactic Acid Fermentation of Black Soldier Fly Prepupae and Related By-Products. Microorganisms. 2020; 8(11):1785. https://doi.org/10.3390/microorganisms8111785
Chicago/Turabian StyleHadj Saadoun, Jasmine, Anna Valentina Luparelli, Augusta Caligiani, Laura Ioana Macavei, Lara Maistrello, Erasmo Neviani, Gianni Galaverna, Stefano Sforza, and Camilla Lazzi. 2020. "Antimicrobial Biomasses from Lactic Acid Fermentation of Black Soldier Fly Prepupae and Related By-Products" Microorganisms 8, no. 11: 1785. https://doi.org/10.3390/microorganisms8111785
APA StyleHadj Saadoun, J., Luparelli, A. V., Caligiani, A., Macavei, L. I., Maistrello, L., Neviani, E., Galaverna, G., Sforza, S., & Lazzi, C. (2020). Antimicrobial Biomasses from Lactic Acid Fermentation of Black Soldier Fly Prepupae and Related By-Products. Microorganisms, 8(11), 1785. https://doi.org/10.3390/microorganisms8111785