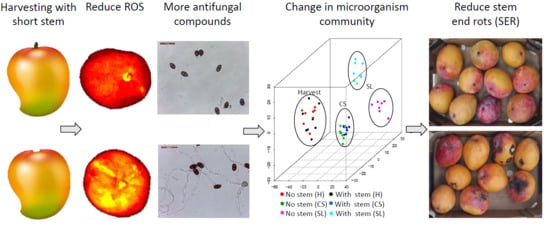
Harvesting Mango Fruit with a Short Stem-End Altered Endophytic Microbiome and Reduce Stem-End Rot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Harvesting Practice, and Storage Conditions
2.2. Evaluation of Fruit Quality Parameters in Mango Fruit
2.3. Decay Evaluation of Mango Fruit
2.4. Fungal Material
2.5. Preparation of Mango Stem-End and Peel Extracts for Antifungal Activity
2.6. Effect of Mango Stem-End Extract on Fungal Growth in Plates
2.7. Effect of Mango Stem-End and Peel Extracts on Fungal Growth in 96 Well-Plates
2.8. Effect of Mango Stem-End and Peel Extracts on Conidia Germination
2.9. The Whole Fruit ROS Level
2.10. Antioxidant Activity
2.11. Microbiome Experimental Design
2.11.1. DNA Extraction
2.11.2. Amplification and Sequencing
2.11.3. Data Analysis and Statistics
3. Results
3.1. Harvesting Mango with Short Stem-End Reduce Postharvest Decays
3.2. Stem-End Endophytic Microbiome Modulations during Storage of Fruit Harvested with or without Stem
3.3. Microorganismal Community’s Changes in Mango that were Harvested with or without Stem-Ends during Storage
3.4. Pathogenic Fungi Dynamics in Mango Stem-End
3.5. Mango without Stem increased Fruit ROS Level and Possess Low Antioxidant Activity
3.6. Mango Stem-End Extract inhibits Pathogenic Fungal Growth
4. Discussion
4.1. Effect of Harvesting Mango with Short Stem-End on SER Development during Storage
4.2. Effect of Harvesting Mango with Short Stem-End on Microbiome Dynamics during Storage
4.3. Antifungal and Antioxidant Properties of Mango Stem-End
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bompard, J. Taxonomy and systematics. In The Mango: Botany, Production and Uses; CAB International: Wallingford, UK, 2009; pp. 19–41. [Google Scholar]
- Johnson, G.I.; Mead, A.J.; Cooke, A.W.; Dean, J.R. Mango stem end rot pathogens-Infection levels between flowering and harvest. Ann. Appl. Biol. 1991, 119, 465–473. [Google Scholar] [CrossRef]
- Alkan, N.; Kumar, P. Postharvest storage management of mango fruit. Achiev. Sustain. Cultiv. Mango; Galán SaúcoV. LuP. Eds 2018, 377–402. [Google Scholar]
- Johnson, G.; Mead, A.; Cooke, A.; Dean, J. Mango stem end rot pathogens--Fruit infection by endophytic colonisation of the inflorescence and pedicel. Ann. Appl. Biol. 1992, 120, 225–234. [Google Scholar] [CrossRef]
- Galsurker, O.; Diskin, S.; Maurer, D.; Feygenberg, O.; Alkan, N. Fruit stem-end rot. Horticulturae 2018, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Diskin, S.; Feygenberg, O.; Maurer, D.; Droby, S.; Prusky, D.; Alkan, N. Microbiome alterations are correlated with occurrence of postharvest stem-end rot in mango fruit. Phytobiomes 2017, 1, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Alkan, N.; Fortes, A.M. Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front. Plant. Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [Green Version]
- Joel, D. The duct systems of the base and stalk of the mango fruit. Bot. Gaz. 1981, 142, 329–333. [Google Scholar] [CrossRef]
- Hassan, M.; Irving, D.; Dann, E.; Coates, L.; Hofman, P. Sap properties and alk (en) ylresorcinol concentrations in Australian—grown mangoes. Ann. Appl. Biol. 2009, 154, 419–427. [Google Scholar] [CrossRef]
- Karunanayake, L.C.; Adikaram, N.; Kumarihamy, B.M.; Bandara, B.R.; Abayasekara, C. Role of antifungal gallotannins, resorcinols and chitinases in the constitutive defence of immature mango (Mangifera indica L.) against Colletotrichum gloeosporioides. J. Phytopathol. 2011, 159, 657–664. [Google Scholar] [CrossRef]
- Hassan, M.; Dann, E.; Irving, D.; Coates, L. Concentrations of constitutive alk (en) ylresorcinols in peel of commercial mango varieties and resistance to postharvest anthracnose. Physiol. Mol. Plant. Pathol. 2007, 71, 158–165. [Google Scholar] [CrossRef]
- Karunanayake, K.; Sinniah, G.; Adikaram, N.; Abayasekara, C.; Wijayasekara, D. Retention of latex at harvest, enhanced mango (Mangifera indica L.) fruit resistance and reduced anthracnose and stem-end rot. Australas. Plant. Pathol. 2015, 44, 113–119. [Google Scholar] [CrossRef]
- Sangchote, S. Botryodiplodia stem end rot of mango and its control. In Proceedings of the III International Mango Symposium 291, Darwin, Australia, 1 June 1991; pp. 296–303. [Google Scholar]
- Gatto, M.A.; Ippolito, A.; Linsalata, V.; Cascarano, N.A.; Nigro, F.; Vanadia, S.; Di Venere, D. Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol. Technol. 2011, 61, 72–82. [Google Scholar] [CrossRef]
- Cheung, L.; Cheung, P.C.; Ooi, V.E. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Bybee, S.M.; Bracken-Grissom, H.; Haynes, B.D.; Hermansen, R.A.; Byers, R.L.; Clement, M.J.; Udall, J.A.; Wilcox, E.R.; Crandall, K.A. Targeted amplicon sequencing (TAS): A scalable next-gen approach to multilocus, multitaxa phylogenetics. Genome Biol. Evol. 2011, 3, 1312–1323. [Google Scholar] [CrossRef] [Green Version]
- De Cárcer, D.A.; Denman, S.E.; McSweeney, C.; Morrison, M. Strategy for modular tagged high-throughput amplicon sequencing. Appl. Environ. Microbiol. 2011, 77, 6310–6312. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.P.; Peay, K.G. Sequence depth, not PCR replication, improves ecological inference from next generation DNA sequencing. PLoS ONE 2014, 9, e90234. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T. The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. CoNet app: Inference of biological association networks using Cytoscape. F1000Research 2016, 5. [Google Scholar]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meah, M. Mode of infection of mango stem-end rot pathogen Botryodiplodia theobromae Pat. Bangladesh J. Bot. 1993. [Google Scholar]
- Sharma, R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Malacrinò, A.; Wisniewski, M.; Cacciola, S.O.; Schena, L. Metabarcoding: A powerful tool to investigate microbial communities and shape future plant protection strategies. Biol. Control. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Do Carmo Teixeira, D.; Saillard, C.; Eveillard, S.; Danet, J.L.; da Costa, P.I.; Ayres, A.J.; Bove, J. ‘Candidatus Liberibacter americanus’, associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Int. J. Syst. Evol. Microbiol. 2005, 55, 1857–1862. [Google Scholar] [CrossRef] [Green Version]
- Karunanayake, K.; Sinniah, G.; Adikaram, N.; Abayasekara, C. Cultivar differences in antifungal activity and the resistance to postharvest anthracnose and stem-end rot in mango (Mangifera indica L.). Australas. Plant. Pathol. 2014, 43, 151–159. [Google Scholar] [CrossRef]
- Adikaram, N.; Karunanayake, C.; Abayasekara, C. The role of pre-formed antifungal substances in the resistance of fruits to postharvest pathogens. In Postharvest Pathology; Springer: Dordrecht, The Netherlands, 2009; pp. 1–11. [Google Scholar]
- Cojocaru, M.; Droby, S.; Glotter, E.; Goldman, A.; Gottlieb, H.E.; Jacoby, B.; Prusky, D. 5-(12-Heptadecenyl)-resorcinol, the major component of the antifungal activity in the peel of mango fruit. Phytochemistry 1986, 25, 1093–1095. [Google Scholar] [CrossRef]
- Droby, S.; Prusky, D.; Jacoby, B.; Goldman, A. Presence of antifungal compounds in the peel of mango fruits and their relation to latent infections of Alternaria alternata. Physiol. Mol. Plant. Pathol. 1986, 29, 173–183. [Google Scholar] [CrossRef]
- Prusky, D.; Plumbley, R. Quiescent infections of Colletotrichum in tropical and subtropical fruits. Quiescent Infect. Colletotrichum Trop. Subtrop. Fruits. 1992, 289–307. [Google Scholar]
- Karunanayake, K. Natural defence mechanisms in mango fruit and their potential in management of postharvest diseases. Ph.D. Thesis, University of Peradeniya, Sri Lanka, 2008. [Google Scholar]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Dickman, M.B.; Fluhr, R. Centrality of host cell death in plant-microbe interactions. Annu. Rev. Phytopathol. 2013, 51, 543–570. [Google Scholar] [CrossRef]






| 2015 | 2016 | 2017 | |||||
|---|---|---|---|---|---|---|---|
| Storage Time | No Stem | With Stem | No Stem | With Stem | No Stem | With Stem | |
| TSS (%) | CS | 11.7 ± 0.30 | 13.7 ± 0.55 | 12.7 + 0.4 | 12.8 + 0.5 | 12 + 0.75 | 11.8 + 0.46 |
| SL | 14.5 ± 0.35 | 14.2 ± 0.38 | 12.1 + 0.6 | 11.8 + 0.3 | 12.9 + 0.22 | 12.2 + 0.18 | |
| Acidity (%) | CS | 0.90 ± 0.04 | 0.95 + 0.02 | 0.8 + 0.06 | 1 + 0.03 | 0.6 + 0.01 | 0.5 + 0.02 |
| SL | 0.27 ± 0.06 | 0.33 ± 0.01 | 0.47 + 0.03 | 0.5 + 0.03 | 0.3 + 0.05 | 0.2 + 0.04 | |
| Color change (index 1–10) | CS | 3.2 ± 0.1 | 2.8 ± 0.1 | 2.6 + 0.2 | 2.3 + 0.1 | 4 + 0.19 | 3.3 + 0.27 |
| SL | 7.9 ± 0.5 | 7.7 ± 0.6 | 8.7 + 0.4 | 9.9 + 0.1 | 9.5 + 0.11 | 9.2 + 0.13 | |
| Firmness (index 1–10) | CS | 10 ± 0.1 | 10 ± 0.0 | 9.9 + 0.1 | 10 + 0.1 | 9.6 + 0.2 | 10 + 0.0 |
| SL | 5.4 ± 0.2 | 5.2 ± 0.2 | 3.8 + 0.2 | 4 + 0.0 | 3.5 + 0.5 | 3.7 + 0.3 | |
| SER (%) | CS | 0 | 0 | 12.0 + 2.7 | 0 | 0 | 0 |
| SL | 0 | 0 | 27.3 + 6.7 | 0 * | 8.1 + 2.6 | 0 | |
| Prolong SL | 82.3 + 8.3 | 29.9 + 7 * | 72.2 + 14.7 | 31 + 10.5 * | 23.8 + 1.3 | 0 * | |
| Fungal ITS | |||
| Treatment | Comparison | H | p-value |
| With stem | Harvest/CS/SL | 11.29 | 0.0007 |
| Without stem | Harvest/CS/SL | 10.6 | 0.0011 |
| Harvest | With stem/without stem | 0.71 | 0.401 |
| CS | With stem/without stem | 4.41 | 0.036 |
| SL | With stem/without stem | 9.93 | 0.001 |
| Bacterial 16S | |||
| Treatment | Comparison | H | p-value |
| With stem | Harvest/CS/SL | 9.28 | 0.002 |
| No stem | Harvest/CS/SL | 0.54 | 0.462 |
| Harvest | With stem/No stem | 2.82 | 0.09 |
| CS | With stem/No stem | 11.29 | 0.0007 |
| SL | With stem/No stem | 0 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galsurker, O.; Diskin, S.; Duanis-Assaf, D.; Doron-Faigenboim, A.; Maurer, D.; Feygenberg, O.; Alkan, N. Harvesting Mango Fruit with a Short Stem-End Altered Endophytic Microbiome and Reduce Stem-End Rot. Microorganisms 2020, 8, 558. https://doi.org/10.3390/microorganisms8040558
Galsurker O, Diskin S, Duanis-Assaf D, Doron-Faigenboim A, Maurer D, Feygenberg O, Alkan N. Harvesting Mango Fruit with a Short Stem-End Altered Endophytic Microbiome and Reduce Stem-End Rot. Microorganisms. 2020; 8(4):558. https://doi.org/10.3390/microorganisms8040558
Chicago/Turabian StyleGalsurker, Ortal, Sonia Diskin, Danielle Duanis-Assaf, Adi Doron-Faigenboim, Dalia Maurer, Oleg Feygenberg, and Noam Alkan. 2020. "Harvesting Mango Fruit with a Short Stem-End Altered Endophytic Microbiome and Reduce Stem-End Rot" Microorganisms 8, no. 4: 558. https://doi.org/10.3390/microorganisms8040558
APA StyleGalsurker, O., Diskin, S., Duanis-Assaf, D., Doron-Faigenboim, A., Maurer, D., Feygenberg, O., & Alkan, N. (2020). Harvesting Mango Fruit with a Short Stem-End Altered Endophytic Microbiome and Reduce Stem-End Rot. Microorganisms, 8(4), 558. https://doi.org/10.3390/microorganisms8040558