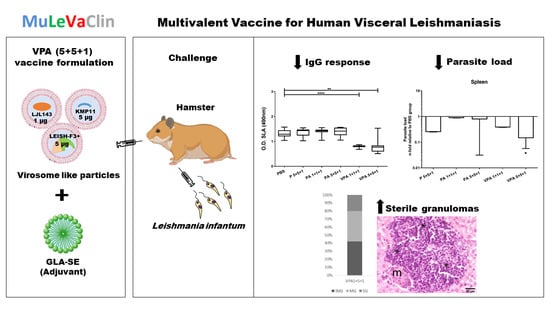
Protective Efficacy in a Hamster Model of a Multivalent Vaccine for Human Visceral Leishmaniasis (MuLeVaClin) Consisting of the KMP11, LEISH-F3+, and LJL143 Antigens in Virosomes, Plus GLA-SE Adjuvant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals, Parasites, Antigens, and Adjuvant
2.3. Virosome-Based Antigens
2.4. Immunization and Infection
2.5. Whole Blood Collection
2.6. Cell Proliferation Assay
2.7. Humoral Response Analysis
2.8. Parasite Load Quantification by PCR
2.9. Histopathology
2.10. Statistical Analysis
3. Results
3.1. Antigenicity of the Different Vaccine Formulations
3.2. Immune Responses in Vaccinated Hamsters after L. infantum Infection
3.3. The Complete Vaccine Formulation Confers Protection against L. infantum Infection in Hamsters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Bottazzi, M.E.; Strych, U. New Vaccines for the World’s Poorest People. Annu. Rev. Med. 2016, 67, 405–417. [Google Scholar] [CrossRef]
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Leishmaniasis Update, 2006–2015: A Turning Point in Leishmaniasis Surveillance. WHO, Diseases DoCoNT; 2017 Contract No. 92. Available online: https://www.who.int/publications/i/item/who-wer9238 (accessed on 11 December 2020).
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Hefnawy, A.; Berg, M.; Dujardin, J.C.; De Muylder, G. Exploiting Knowledge on Leishmania Drug Resistance to Support the Quest for New Drugs. Trends Parasitol. 2017, 33, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, G.; Nieto, J.; Foglia Manzillo, V.; Cappiello, S.; Fiorentino, E.; Di Muccio, T.; Scalone, A.; Moreno, J.; Chicharro, C.; Carrillo, E.; et al. A randomised, double-blind, controlled efficacy trial of the LiESP/QA-21 vaccine in naive dogs exposed to two Leishmania infantum transmission seasons. PLoS Negl. Trop. Dis. 2014, 8, e3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, C.B.; Junior, J.T.; de Jesus, C.; Souza, B.M.; Larangeira, D.F.; Fraga, D.B.; Tavares Veras, P.S.; Barrouin-Melo, S.M. Comparison of two commercial vaccines against visceral leishmaniasis in dogs from endemic areas: IgG, and subclasses, parasitism, and parasite transmission by xenodiagnosis. Vaccine 2014, 32, 1287–1295. [Google Scholar] [CrossRef]
- Fernandez Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Maranon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend(R) against canine leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Moreno, J. Assessment of Vaccine-Induced Immunity Against Canine Visceral Leishmaniasis. Front. Vet. Sci. 2019, 6, 168. [Google Scholar] [CrossRef]
- Velez, R.; Gallego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef] [Green Version]
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against Leishmania under current research. Expert Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Bhaumik, S.; Basu, J.M.; Naskar, K.; De, T.; Roy, S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: Evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J. Immunol. 2005, 174, 7160–7171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Bernal, G.; Horcajo, P.; Orden, J.A.; Ruiz-Santa-Quiteria, J.A.; De La Fuente, R.; Ordóñez-Gutiérrez, L.; Martínez-Rodrigo, A.; Mas, A.; Carrión, J. HisAK70: Progress towards a vaccine against different forms of leishmaniosis. Parasit. Vectors 2015, 8, 629. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Rodrigo, A.; Dias, D.S.; Ribeiro, P.A.F.; Roatt, B.M.; Mas, A.; Carrion, J.; Coelho, E.A.F.; Domínguez-Bernal, G. Immunization with the HisAK70 DNA Vaccine Induces Resistance against Leishmania amazonensis Infection in BALB/c Mice. Vaccines 2019, 7, 183. [Google Scholar] [CrossRef] [Green Version]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef] [Green Version]
- Coler, R.N.; Duthie, M.S.; Hofmeyer, K.A.; Guderian, J.; Jayashankar, L.; Vergara, J.; Rolf, T.; Misquith, A.; Laurance, J.D.; Raman, V.S.; et al. From mouse to man: Safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin. Transl. Immunol. 2015, 4, e35. [Google Scholar] [CrossRef]
- Duthie, M.S.; Pereira, L.; Favila, M.; Hofmeyer, K.A.; Reed, S.J.; Metangmo, S.; Townsend, S.; Laurance, J.D.; Picone, A.; Misquith, A.; et al. A defined subunit vaccine that protects against vector-borne visceral leishmaniasis. NPJ Vaccines 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Van Hoeven, N.; MacMillen, Z.; Picone, A.; Mohamath, R.; Erasmus, J.; Hsu, F.C.; Stinchcomb, D.T.; Reed, S.G. Heterologous Immunization With Defined RNA and Subunit Vaccines Enhances T Cell Responses That Protect Against Leishmania donovani. Front. Immunol. 2018, 9, 2420. [Google Scholar] [CrossRef] [Green Version]
- Abdeladhim, M.; Kamhawi, S.; Valenzuela, J.G. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect. Genet. Evol. 2014, 28, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Lestinova, T.; Rohousova, I.; Sima, M.; de Oliveira, C.I.; Volf, P. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017, 11, e0005600. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Teixeira, C.; Teixeira, M.J.; Oliveira, F.; Menezes, M.J.; Silva, C.; de Oliveira, C.I.; Miranda, J.C.; Elnaiem, D.E.; Kamhawi, S.; et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc. Natl. Acad. Sci. USA 2008, 105, 7845–7850. [Google Scholar] [CrossRef] [Green Version]
- Collin, N.; Gomes, R.; Teixeira, C.; Cheng, L.; Laughinghouse, A.; Ward, J.M.; Elnaiem, D.E.; Fischer, L.; Valenzuela, J.G.; Kamhawi, S. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009, 5, e1000441. [Google Scholar] [CrossRef]
- Cunha, J.M.; Abbehusen, M.; Suarez, M.; Valenzuela, J.; Teixeira, C.R.; Brodskyn, C.I. Immunization with LJM11 salivary protein protects against infection with Leishmania braziliensis in the presence of Lutzomyia longipalpis saliva. Acta Trop. 2018, 177, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, J.A.; Dey, R.; Davenport, D.; Abdeladhim, M.; Meneses, C.; Oliveira, F.; Kamhawi, S.; Valenzuela, J.G.; Gannavaram, S.; Nakhasi, H.L. Intradermal Immunization of Leishmania donovani Centrin Knock-Out Parasites in Combination with Salivary Protein LJM19 from Sand Fly Vector Induces a Durable Protective Immune Response in Hamsters. PLoS Negl. Trop. Dis. 2016, 10, e0004322. [Google Scholar] [CrossRef]
- Cecilio, P.; Perez-Cabezas, B.; Fernandez, L.; Moreno, J.; Carrillo, E.; Requena, J.M.; Fichera, E.; Reed, S.G.; Coler, R.N.; Kamhawi, S.; et al. Pre-clinical antigenicity studies of an innovative multivalent vaccine for human visceral leishmaniasis. PLoS Negl. Trop. Dis. 2017, 11, e0005951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbehusen, M.M.C.; Cunha, J.; Suarez, M.S.; Teixeira, C.; Almeida, V.D.A.; Pereira, L.D.S.; Bordoni, M.; Gil-Santana, L.; Solcà, M.D.S.; Fraga, D.B.M.; et al. Immunization of Experimental Dogs With Salivary Proteins From Lutzomyia longipalpis, Using DNA and Recombinant Canarypox Virus Induces Immune Responses Consistent With Protection Against Leishmania infantum. Front. Immunol. 2018, 9, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, V.S.; Duthie, M.S.; Fox, C.B.; Matlashewski, G.; Reed, S.G. Adjuvants for Leishmania vaccines: From models to clinical application. Front. Immunol. 2012, 3, 144. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.B.; Baldwin, S.L.; Duthie, M.S.; Reed, S.G.; Vedvick, T.S. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine 2011, 29, 9563–9572. [Google Scholar] [CrossRef] [Green Version]
- Coler, R.N.; Day, T.A.; Ellis, R.; Piazza, F.M.; Beckmann, A.M.; Vergara, J.; Rolf, T.; Lu, L.; Alter, G.; Hokey, D.; et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines 2018, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Glück, R.; Metcalfe, I.C. Novel approaches in the development of immunopotentiating reconstituted influenza virosomes as efficient antigen carrier systems. Vaccine 2003, 21, 611–615. [Google Scholar] [CrossRef]
- Kammer, A.R.; Amacker, M.; Rasi, S.; Westerfeld, N.; Gremion, C.; Neuhaus, D.; Zurbriggen, R. A new and versatile virosomal antigen delivery system to induce cellular and humoral immune responses. Vaccine 2007, 25, 7065–7074. [Google Scholar] [CrossRef] [PubMed]
- Cusi, M.G. Applications of influenza virosomes as a delivery system. Hum. Vaccine 2006, 2, 1–7. [Google Scholar] [CrossRef]
- Bungener, L.; Idema, J.; ter Veer, W.; Huckriede, A.; Daemen, T.; Wilschut, J. Virosomes in vaccine development: Induction of cytotoxic T lymphocyte activity with virosome-encapsulated protein antigens. J. Liposome Res. 2002, 12, 155–163. [Google Scholar] [CrossRef]
- Bungener, L.; Serre, K.; Bijl, L.; Leserman, L.; Wilschut, J.; Daemen, T.; Machy, P. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine 2002, 20, 2287–2295. [Google Scholar] [CrossRef]
- Blackwell, J.M.; Roberts, B.; Alexander, J. Response of BALB/c mice to leishmanial infection. Curr. Top. Microbiol. Immunol. 1985, 122, 97–106. [Google Scholar] [CrossRef]
- Escobar, P.; Yardley, V.; Croft, S.L. Activities of hexadecylphosphocholine (miltefosine), AmBisome, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrob. Agents Chemother. 2001, 45, 1872–1875. [Google Scholar] [CrossRef] [Green Version]
- Maroof, A.; Brown, N.; Smith, B.; Hodgkinson, M.R.; Maxwell, A.; Losch, F.O.; Fritz, U.; Walden, P.; Lacey, C.N.J.; Smith, D.F.; et al. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J. Infect. Dis. 2012, 205, 853–863. [Google Scholar] [CrossRef]
- Stager, S.; Alexander, J.; Carter, K.C.; Brombacher, F.; Kaye, P.M. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect. Immun. 2003, 71, 4804–4807. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Rai, A.K. Hamster, a close model for visceral leishmaniasis: Opportunities and challenges. Parasite Immunol. 2020, 42, e12768. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Domínguez-Bernal, G.; Orden, J.A.; De La Fuente, R.; Madrid-Elena, N.; Carrión, J. Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model. Vet. Res. 2011, 42, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo, E.; Fernandez, L.; Ibarra-Meneses, A.V.; Santos, M.L.B.; Nico, D.; de Luca, P.M.; Correa, C.B.; de Almeida, R.P.; Moreno, J.; Palatnik-de-Sousa, C.B. F1 Domain of the Leishmania (Leishmania) donovani Nucleoside Hydrolase Promotes a Th1 Response in Leishmania (Leishmania) infantum Cured Patients and in Asymptomatic Individuals Living in an Endemic Area of Leishmaniasis. Front. Immunol. 2017, 8, 750. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.A.; Van Roey, G.A.; Tregoning, J.S.; Moutaftsi, M.; Coler, R.N.; Windish, H.P.; Reed, S.G.; Carter, D.; Shattock, R.J. Glucopyranosyl Lipid Adjuvant (GLA), a Synthetic TLR4 agonist, promotes potent systemic and mucosal responses to intranasal immunization with HIVgp140. PLoS ONE 2012, 7, e41144. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, E.; Jimenez, M.A.; Sanchez, C.; Cunha, J.; Martins, C.M.; da Paixao Seva, A.; Moreno, J. Protein malnutrition impairs the immune response and influences the severity of infection in a hamster model of chronic visceral leishmaniasis. PLoS ONE 2014, 9, e89412. [Google Scholar] [CrossRef]
- Santarem, N.; Cunha, J.; Silvestre, R.; Silva, C.; Moreira, D.; Ouellette, M.; Cordeiro-da-Silva, A. The impact of distinct culture media in Leishmania infantum biology and infectivity. Parasitology 2014, 141, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Heimann, M.; Kasermann, H.P.; Pfister, R.; Roth, D.R.; Burki, K. Blood collection from the sublingual vein in mice and hamsters: A suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Lab Anim. 2009, 43, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambrook, J.; Russell, D.W. The Condensed Protocols from Molecular Cloning: A Laboratory Manual; Inglis, J., Ed.; CSHL Press: New York, NY, USA, 2006. [Google Scholar]
- Cunha, J.; Carrillo, E.; Sanchez, C.; Cruz, I.; Moreno, J.; Cordeiro-da-Silva, A. Characterization of the biology and infectivity of Leishmania infantum viscerotropic and dermotropic strains isolated from HIV+ and HIV- patients in the murine model of visceral leishmaniasis. Parasit. Vectors 2013, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.; Myburgh, E.; Gao, M.Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 2001, 82, 249–267. [Google Scholar] [CrossRef]
- Salguero, F.J.; Garcia-Jimenez, W.L.; Lima, I.; Seifert, K. Histopathological and immunohistochemical characterisation of hepatic granulomas in Leishmania donovani-infected BALB/c mice: A time-course study. Parasit. Vectors 2018, 11, 73. [Google Scholar] [CrossRef]
- Mas, A.; Martinez-Rodrigo, A.; Orden, J.A.; Molina, R.; Jimenez, M.; Jimenez, M.A.; Carrión, J.; Domínguez-Bernal, G. Properties of virulence emergence of Leishmania infantum isolates from Phlebotomus perniciosus collected during the human leishmaniosis outbreak in Madrid, Spain. Hepatic histopathology and immunological parameters as virulence markers in the mouse model. Transbound. Emerg. Dis. 2020, 68, 704–714. [Google Scholar] [CrossRef]
- Moser, C.; Amacker, M.; Zurbriggen, R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev. Vaccines 2011, 10, 437–446. [Google Scholar] [CrossRef]
- Sirima, S.B.; Richert, L.; Chene, A.; Konate, A.T.; Campion, C.; Dechavanne, S.; Semblat, J.P.; Benhamouda, N.; Bahuaud, M.; Loulergue, P.; et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: A first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis. 2020, 20, 585–597. [Google Scholar] [CrossRef]
- Duthie, M.S.; Frevol, A.; Day, T.; Coler, R.N.; Vergara, J.; Rolf, T.; Sagawa, Z.K.; Marie Beckmann, A.; Casper, C.; Reed, S.G. A phase 1 antigen dose escalation trial to evaluate safety, tolerability and immunogenicity of the leprosy vaccine candidate LepVax (LEP-F1 + GLA-SE) in healthy adults. Vaccine 2020, 38, 1700–1707. [Google Scholar] [CrossRef]
- Sunay, M.M.E.; Martins, K.A.O.; Steffens, J.T.; Gregory, M.; Vantongeren, S.A.; Van Hoeven, N.; Garnes, P.G.; Bavari, S. Glucopyranosyl lipid adjuvant enhances immune response to Ebola virus-like particle vaccine in mice. Vaccine 2019, 37, 3902–3910. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.C.; Bertholet, S.; Lawyer, P.G.; Charmoy, M.; Romano, A.; Ribeiro-Gomes, F.L.; Stamper, L.W.; Sacks, D.L. Evaluation of recombinant Leishmania polyprotein plus glucopyranosyl lipid A stable emulsion vaccines against sand fly-transmitted Leishmania major in C57BL/6 mice. J. Immunol. 2012, 189, 4832–4841. [Google Scholar] [CrossRef] [Green Version]
- Nickol, A.D.; Bonventre, P.F. Immunosuppression associated with visceral leishmaniasis of hamsters. Parasite Immunol. 1985, 7, 439–449. [Google Scholar] [CrossRef]
- Kamhawi, S.; Aslan, H.; Valenzuela, J.G. Vector saliva in vaccines for visceral leishmaniasis: A brief encounter of high consequence? Front. Public Health 2014, 2, 99. [Google Scholar] [CrossRef] [Green Version]
- Loria-Cervera, E.N.; Andrade-Narvaez, F.J. Animal models for the study of leishmaniasis immunology. Rev. Inst. Med. Trop. 2014, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Anton, M.D.; Grau, M.; Corral, M.J.; Olias-Molero, A.I.; Alunda, J.M. Efficient infection of hamster with Leishmania donovani by retro-orbital inoculation. Virulence 2019, 10, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Moreira, N.; Vitoriano-Souza, J.; Roatt, B.M.; Vieira, P.M.; Coura-Vital, W.; Cardoso, J.M.; Rezende, M.T.; Ker, H.G.; Giunchetti, R.C.; Carneiro, C.M.; et al. Clinical, hematological and biochemical alterations in hamster (Mesocricetus auratus) experimentally infected with Leishmania infantum through different routes of inoculation. Parasit. Vectors 2016, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Moreira, N.; Vitoriano-Souza, J.; Roatt, B.M.; Vieira, P.M.; Ker, H.G.; de Oliveira Cardoso, J.M.; Giunchetti, R.C.; Carneiro, C.M.; de Lana, M.; Reis, A.B. Parasite burden in hamsters infected with two different strains of Leishmania (Leishmania) infantum: “Leishman Donovan units” versus real-time PCR. PLoS ONE 2012, 7, e47907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Martin, I.; Jimenez, M.; Gonzalez, E.; Eguiluz, C.; Molina, R. Natural transmission of Leishmania infantum through experimentally infected Phlebotomus perniciosus highlights the virulence of Leishmania parasites circulating in the human visceral leishmaniasis outbreak in Madrid, Spain. Vet. Res. 2015, 46, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena, J.M.; Soto, M.; Doria, M.D.; Alonso, C. Immune and clinical parameters associated with Leishmania infantum infection in the golden hamster model. Vet. Immunol. Immunopathol. 2000, 76, 269–281. [Google Scholar] [CrossRef]
- Deak, E.; Jayakumar, A.; Cho, K.W.; Goldsmith-Pestana, K.; Dondji, B.; Lambris, J.D.; McMahon-Pratt, D. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur. J. Immunol. 2010, 40, 1355–1368. [Google Scholar] [CrossRef]
- Miles, S.A.; Conrad, S.M.; Alves, R.G.; Jeronimo, S.M.; Mosser, D.M. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005, 201, 747–754. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cordeiro-da-Silva, A.; Laforge, M.; Silvestre, R.; Estaquier, J. Regulation of immunity during visceral Leishmania infection. Parasit. Vectors 2016, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, P.; Goncalves, R.; Mosser, D.M. The influence of IgG density and macrophage Fc (gamma) receptor cross-linking on phagocytosis and IL-10 production. Immunol. Lett. 2010, 133, 70–77. [Google Scholar] [CrossRef] [Green Version]





| Group | Components |
|---|---|
| PBS | PBS 1 |
| P 5+5+1 | LEISH-F3 + (5 µg)/KMP11 (5 µg)/LJL143 (1 ug) |
| PA 1+1+1 | LEISH-F3 + (1 µg)/KMP11 (1 µg)/LJL143 (1 µg)/GLA-SE (1 µg) |
| PA 5+5+1 | LEISH-F3 + (5 µg)/KMP11 (5 µg)/LJL143 (1 µg)/GLA-SE (1 µg) |
| VPA 1+1+1 | VS- LEISH-F3 + (1 µg)/VS-KMP11 (1 µg)/VS-LJL143 (1 µg)/GLA-SE (1 µg) |
| VPA 5+5+1 | VS-LEISH-F3 + (5 µg)/VS-KMP11 (5 µg)/VS-LJL143 (1 µg)/GLA-SE (1 µg) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, L.; Solana, J.C.; Sánchez, C.; Jiménez, M.Á.; Requena, J.M.; Coler, R.; Reed, S.G.; Valenzuela, J.G.; Kamhawi, S.; Oliveira, F.; et al. Protective Efficacy in a Hamster Model of a Multivalent Vaccine for Human Visceral Leishmaniasis (MuLeVaClin) Consisting of the KMP11, LEISH-F3+, and LJL143 Antigens in Virosomes, Plus GLA-SE Adjuvant. Microorganisms 2021, 9, 2253. https://doi.org/10.3390/microorganisms9112253
Fernández L, Solana JC, Sánchez C, Jiménez MÁ, Requena JM, Coler R, Reed SG, Valenzuela JG, Kamhawi S, Oliveira F, et al. Protective Efficacy in a Hamster Model of a Multivalent Vaccine for Human Visceral Leishmaniasis (MuLeVaClin) Consisting of the KMP11, LEISH-F3+, and LJL143 Antigens in Virosomes, Plus GLA-SE Adjuvant. Microorganisms. 2021; 9(11):2253. https://doi.org/10.3390/microorganisms9112253
Chicago/Turabian StyleFernández, Laura, Jose Carlos Solana, Carmen Sánchez, Mª Ángeles Jiménez, Jose M. Requena, Rhea Coler, Steven G. Reed, Jesus G. Valenzuela, Shaden Kamhawi, Fabiano Oliveira, and et al. 2021. "Protective Efficacy in a Hamster Model of a Multivalent Vaccine for Human Visceral Leishmaniasis (MuLeVaClin) Consisting of the KMP11, LEISH-F3+, and LJL143 Antigens in Virosomes, Plus GLA-SE Adjuvant" Microorganisms 9, no. 11: 2253. https://doi.org/10.3390/microorganisms9112253
APA StyleFernández, L., Solana, J. C., Sánchez, C., Jiménez, M. Á., Requena, J. M., Coler, R., Reed, S. G., Valenzuela, J. G., Kamhawi, S., Oliveira, F., Fichera, E., Glueck, R., Bottazzi, M. E., Gupta, G., Cecilio, P., Pérez-Cabezas, B., Cordeiro-da-Silva, A., Gradoni, L., Carrillo, E., & Moreno, J. (2021). Protective Efficacy in a Hamster Model of a Multivalent Vaccine for Human Visceral Leishmaniasis (MuLeVaClin) Consisting of the KMP11, LEISH-F3+, and LJL143 Antigens in Virosomes, Plus GLA-SE Adjuvant. Microorganisms, 9(11), 2253. https://doi.org/10.3390/microorganisms9112253