Bioreactor Co-Cultivation of High Lipid and Carotenoid Producing Yeast Rhodotorula kratochvilovae and Several Microalgae under Stress
Abstract
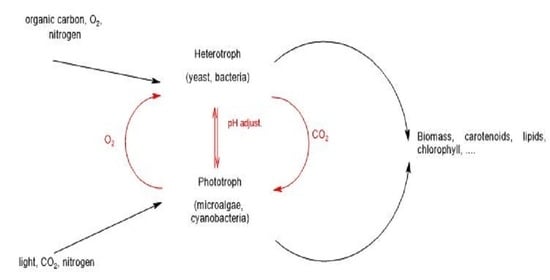
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Microalgae Strains
2.3. Media Preparation
2.4. Co-Cultivation Experimental Scheme
- Yeast growth curve and production on mineral BBM medium with different carbon and nitrogen sources in 4-day experiments.
- Possible mixotrophy of selected strains of microalgae focusing on the utilization of glucose or glycerol in BBM media.
- Small-scale co-cultivation experiments in Erlenmeyer flasks to determine the compatibility of yeasts and microalgae.
- Co-cultivation in aerated round flasks under illumination to confirm small-scale experiments.
- Co-cultivation under controlled conditions in a 3L bioreactor.
2.4.1. Yeasts Cultivation on Mineral BBM Media (1st Phase)
2.4.2. Microalgae Cultivation on BBM Media with an Organic Carbon Source (2nd Phase)
2.4.3. Co-Cultivation of Yeasts and Microalgae in Small Scale (3rd Phase)
2.4.4. Co-Cultivation in an Aerated 1 L round Pyrex Bottle (4th Phase)
2.4.5. Large Scale Bioreactor Co-Cultivation (5th Phase)
2.5. Analytical Methods
2.5.1. Cell Dry Weight
2.5.2. Pigment Analysis
2.5.3. Lipids and Fatty Acids
2.6. Statistical Analysis
3. Results
3.1. Yeast Cultivation on BBM and Standard Media
3.1.1. Biomass Production
3.1.2. Carotenoid, Sterol and Ubiquinone Production
3.1.3. Fatty Acid Profile and Lipid Production
3.2. Microalgae Cultivation on BBM Media
3.3. Small Scale Co-Cultivation of Yeasts and Microalgae
3.3.1. Biomass Production during Small Scale Co-Cultivation
3.3.2. Carotenoid, Chlorophyll, Sterol, and Ubiquinone Production in Co-Culture of R. kratochvilovae
3.3.3. Fatty Acid Profile and Lipid Production in Small Scale Co-Cultivation
3.4. Co-Cultivation of Yeasts and Microalgae in Aerated Flasks
3.4.1. Growth of Co-Culture
3.4.2. Carotenoid, Chlorophyll, Sterols, and Ubiquinone Production in Cultures Grown in Pyrex Flasks
3.4.3. Fatty Acid Profile and Lipid Production in Co-Culture Grown in Pyrex Flasks
3.5. Co-Cultivation of R. kratochvilovae with Microalgae in Laboratory Bioreactor
3.5.1. Biomass Production during Bioreactor Co-Cultivation of Rhodotorula kratochvilovae and Desmodesmus sp.
3.5.2. Cell Morphology in Co-Culture
3.5.3. Production of Pigments, Sterols, and Quinones during Bioreactor Co-Cultivation of Rhodotorula kratochvilovae and Desmodesmus sp.
3.5.4. Production of Lipids and Fatty Acids during Bioreactor Co-Cultivation of Rhodotorula kratochvilovae and Desmodesmus sp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schilling, C.; Steve, W. A Roadmap for Industry to Harness Biotechnology for a More Circular Economy. New Biotechnol. 2021, 60, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chuanglin, F. Circular economy development in China-current situation, evaluation and policy implications. Environ. Impact Assess. Rev. 2020, 84, 106441. [Google Scholar] [CrossRef]
- Yen, H.-W.; Chen, P.-W.; Chen, L.-J. The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour. Technol. 2015, 184, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M. Yeasts. In Encyclopedia of Microbiology; Elsevier: Amsterodam, The Netherlands, 2009; pp. 478–491. ISBN 9780123739445. [Google Scholar]
- Hill, A.E. Brewing Microbiology-Managing Microbes, Ensuring Quality and Valorising Waste; Elsevier: Amsterodam, The Netherlands, 2015; ISBN 978-1-78242-349-2. [Google Scholar]
- Papanikolaou, S.; Dimou, A.; Fakas, S.; Diamantopoulou, P.; Philippoussis, A.; Galiotou-Panayotou, M.; Aggelis, G. Biotechnological conversion of waste cooking olive oil into lipid-rich biomass using Aspergillus and Penicillium strains. J. Appl. Microbiol. 2011, 110, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Kosa, G.; Kohler, A.; Tafintseva, V.; Zimmermann, B.; Forfang, K.; Afseth, N.K.; Tzimorotas, D.; Vuoristo, K.S.; Horn, S.J.; Mounier, J.; et al. Microtiter plate cultivation of oleaginous fungi and monitoring of lipogenesis by high-throughput FTIR spectroscopy. Microb. Cell Factories 2017, 16, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szotkowski, M.; Byrtusova, D.; Haronikova, A.; Vysoka, M.; Rapta, M.; Shapaval, V.; Marova, I. Study of Metabolic Adaptation of Red Yeasts to Waste Animal Fat Substrate. Microorganisms 2019, 7, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latha, B.; Jeevaratman, K.; Murali, H.; Manja, K. Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-PDY from natural source. Indian J. Biotechnol. 2005, 4, 353–357. [Google Scholar]
- Libkind, D.; van Broock, M. Biomass and carotenoid pigment production by patagonian native yeasts. World J. Microbiol. Biotechnol. 2006, 22, 687–692. [Google Scholar] [CrossRef]
- Yurkov, A.; Vustin, M.; Tyaglov, B.; Maksimova, I.; Sineokiy, S. Pigmented basidiomycetous yeasts are a promising source of carotenoids and ubiquinone Q10. Microbiology 2008, 77, 1–6. [Google Scholar] [CrossRef]
- Mannazzu, I.; Landolfo, S.; da Silva, T.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullerman, L.B. SPOILAGE|Fungi in Food—An Overview. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterodam, The Netherlands, 2003; pp. 5511–5522. ISBN 9780122270550. [Google Scholar]
- Santos, F.M.; Gonçalves, A.L.; Pires, J.C.M. Negative emission technologies. In Bioenergy with Carbon Capture and Storage; Elsevier: Amsterodam, The Netherlands, 2019; pp. 1–13. ISBN 9780128162293. [Google Scholar]
- Singh, L.; Chaudhary, G. Liquid Biofuel Production [online]; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; ISBN 978-1-119-45987-3. [Google Scholar]
- Vidyashankar, S.; Ravishankar, G.A. Algae-Based Bioremediation. In Bioremediation and Bioeconomy; Elsevier: Amsterodam, The Netherlands, 2016; pp. 457–493. ISBN 9780128028308. [Google Scholar]
- Liang, Y.; Kashdan, T.; Sterner, C.; Dombrowski, L.; Petrick, I.; Kröger, M.; Höfer, R.; Biorefineries, A. Industrial Biorefineries & White Biotechnology; Elsevier: Amsterodam, The Netherlands, 2015; pp. 35–90. ISBN 9780444634535. [Google Scholar]
- Kitcha, S.; Cheirsilp, B. Enhanced Lipid Production by Co-cultivation and Co-encapsulation of Oleaginous Yeast Trichosporonoides spathulata with Microalgae in Alginate Gel Beads. Appl. Biochem. Biotechnol. 2014, 173, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Byrtusová, D.; Szotkowski, M.; Kouřilová, X.; Rapta, M.; Márová, I. Production of pigments during co-cultivation of red yeasts and green algae. In 46th Annual Meeting on Yeasts; Book of Abstracts 1; Chemický ústav SAV Bratislava, SR: Bratislava, Slovakia, 2019; p. 46. [Google Scholar]
- Gründemann, C.; Garcia-Käufer, M.; Sauer, B.; Scheer, R.; Merdivan, S.; Bettin, P.; Huber, R.; Lindequist, U. Comparative chemical and biological investigations of β-glucan-containing products from Shiitake mushrooms. J. Funct. Foods 2015, 18, 692–702. [Google Scholar]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.; Hernandez, R.; Holmes, W.; Claupein, W. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Jiru, T.; Groenewald, M.; Pohl, C.; Steyn, L.; Kiggundu, N.; Abate, D. Optimization of cultivation conditions for biotechnological production of lipid by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 for biodiesel preparation. 3 Biotech 2017, 7, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrtusová, D.; Shapaval, V.; Holub, J.; Šimanský, S.; Rapta, M.; Szotkowski, M.; Kohler, A.; Márová, I. Revealing the Potential of Lipid and β-Glucans Coproduction in Basidiomycetes Yeast. Microorganisms 2020, 8, 1034. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Gomes, A.S.; Silva, C.M.; Belo, I. Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Yeast Mineral Cultivation Media | ||||||
|---|---|---|---|---|---|---|
| Flask 1. | Flask 2. | Flask 3. | Flask 4. | Flask 5. | Flask 6. | |
| Carbon source | Glucose | Glucose | Glucose | Glycerol | Glycerol | Glycerol |
| Nitrogen source | Urea | (NH4)2SO4 | Yeast autolysate | Urea | (NH4)2SO4 | Yeast autolysate |
| BBM mineral media | ||||||
| Carbon source | Glucose | Glucose | Glucose | Glycerol | Glycerol | Glycerol |
| Nitrogen source | Urea | (NH4)2SO4 | Yeast autolysate | Urea | (NH4)2SO4 | Yeast autolysate |
| Part A: Rhodotorula kratochvilovae Co-Cultivated with Desmodesmus quadricauda | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Betacarotene | Lutein | Torulene | Total Carotenoids | Chlorophyll A | Chlorophyll B | Co Q | Ergosterol |
| R.kratochvilovae | 0.224 ± 0.015 | 0 | 0.706 ± 0.096 | 1.399 ± 0.156 | 0 | 0 | 2.400 ± 0.186 | 5.439 ± 0.239 |
| DQ − C | 1.256 ± 0.135 | 0.271 ± 0.051 | 0 | 4.403 ± 0.236 | 1.255 ± 0.083 | 1.068 ± 0.086 | 3.333 ± 0.198 | 0 |
| DQ + C | 0.780 ± 0.096 | 0.159 ± 0.023 | 0 | 5.491 ± 0.267 | 0.105 ± 0.016 | 0.051 ± 0.008 | 1.888 ± 0.156 | 0 |
| RK+DQ 1:1 | 0.406 ± 0.056 | 0.001 ± 0.001 | 1.367 ± 0.064 | 3.976 ± 0.196 | 0.234 ± 0.013 | 0 | 6.035 ± 0.213 | 5.370 ± 0.256 |
| RK+DQ 1:2 | 2.231 ± 0.136 | 0.136 ± 0.026 | 0.656 ± 0.081 | 11.221 ± 0.362 | 0.097 ± 0.009 | 0.038 ± 0.004 | 3.325 ± 0.106 | 2.954 ± 0.186 |
| RK+DQ 1:4 | 0.592 ± 0.089 | 0.041 ± 0.006 | 1.332 ± 0.046 | 3.837 ± 0.185 | 0.167 ± 0.026 | 0.187 ± 0.021 | 3.452 ± 0.093 | 3.758 ± 0.125 |
| Part B: Rhodotorula kratochvilovae Co-Cultivated with Desmodesmus acutus | ||||||||
| Strain | Betacarotene | Lutein | Torularhodin | Total Carotenoids | Chlorophyll A | Chlorophyll B | Co Q | Ergosterol |
| RK | 0.022 ± 0.008 | 0 | 0.013 ± 0.003 | 0.162 ± 0.033 | 0 | 0 | 0.240 ± 0.018 | 0.544 ± 0.061 |
| RK + DA 1:1 | 0.401 ± 0.064 | 0.026 ± 0.008 | 0.617 ± 0.053 | 2.969 ± 0.126 | 0.518 ± 0.023 | 0.303 ± 0.032 | 3.390 ± 0.136 | 4.634 ± 0.139 |
| RK + DA 1:2 | 1.241 ± 0.093 | 0.103 ± 0.013 | 0.210 ± 0.039 | 3.790 ± 0.157 | 3.176 ± 0.094 | 1.010 ± 0.091 | 4.565 ± 0.183 | 2.638 ± 0.094 |
| RK + DA 1:4 | 0.562 ± 0.066 | 0.057 ± 0.010 | 0.625 ± 0.065 | 3.339 ± 0.113 | 1.106 ± 0.106 | 0.623 ± 0.076 | 1.563 ± 0.121 | 4.196 ± 0.144 |
| Part C: Rhodotorula kratochvilovae Co-Cultivated with Scenedesmus obliquus | ||||||||
| Betacarotene | Lutein | Torularhodin | Total Carotenoids | Chlorophyll A | Chlorophyll B | Coenzyme Q | Ergosterol | |
| RK | 0.224 ± 0.036 | 0 | 0.134 ± 0.023 | 1.621 ± 0.072 | 0 | 0 | 2.400 ± 0.112 | 5.439 ± 0.239 |
| SO − C | 3.344 ± 0.103 | 5.090 ± 0.232 | 0 | 13.063 ± 0.361 | 13.277 ± 0.226 | 7.589 ± 0.245 | 0.263 ± 0.013 | 0 |
| SO + C | 1.811 ± 0.096 | 0.114 ± 0.019 | 0 | 3.293 ± 0.134 | 0.599 ± 0.049 | 0.292 ± 0.054 | 0.841 ± 0.036 | 0 |
| RK + SO 1:1 | 4.660 ± 0.236 | 0.115 ± 0.010 | 0.175 ± 0.023 | 7.458 ± 0.176 | 2.058 ± 0.131 | 0.850 ± 0.068 | 4.733 ± 0.099 | 2.129 ± 0.083 |
| RK + SO 1:2 | 0.571 ± 0.083 | 0.057 ± 0.006 | 0.496 ± 0.061 | 2.756 ± 0.153 | 0.305 ± 0.026 | 0.174 ± 0.012 | 3.309 ± 0.135 | 5.518 ± 0.222 |
| RK + SO 1:4 | 0.836 ± 0.069 | 0.099 ± 0.007 | 0.468 ± 0.043 | 3.409 ± 0.138 | 0.679 ± 0.044 | 0.293 ± 0.033 | 3.448 ± 0.112 | 4.967 ± 0.164 |
| Rhodotorulakratochvilovae + Desmodesmus quadricauda | |||||||
|---|---|---|---|---|---|---|---|
| Time | Lutein | Torularhodin | Betacarotene | Total Carotenoids | Total Chlorophylls | Ergosterol | Ubiquinone |
| T 6 h | 0.097 ± 0.008 | 1.617 ± 0.003 | 0.216 ± 0.007 | 2.113 ± 0.024 | 0.425 ± 0.058 | 5.621 ± 0.036 | 4.445 ± 0.038 |
| T 24 h | 0.128 ± 0.012 | 0.988 ± 0.005 | 0.225 ± 0.010 | 1.484 ± 0.018 | 0.703 ± 0.012 | 2.683 ± 0.042 | 2.058 ± 0.026 |
| T 30 h | 0.061 ± 0.009 | 0.645 ± 0.008 | 0.131 ± 0.008 | 0.960 ± 0.020 | 0.479 ± 0.017 | 1.465 ± 0.040 | 1.253 ± 0.015 |
| T 48 h | 0.061 ± 0.011 | 0.948 ± 0.010 | 0.163 ± 0.017 | 1.293 ± 0.019 | 0.388 ± 0.009 | 3.052 ± 0.038 | 4.785 ± 0.035 |
| T 72 h | 0.048 ± 0.006 | 0.670 ± 0.004 | 0.145 ± 0.006 | 1.050 ± 0.021 | 0.327 ± 0.006 | 2.600 ± 0.028 | 5.409 ± 0.042 |
| T 96 h | 0.007 ± 0.002 | 0.129 ± 0.013 | 0.032 ± 0.016 | 0.291 ± 0.007 | 0.101 ± 0.010 | 0.779 ± 0.013 | 2.650 ± 0.032 |
| T 120 h | 0.039 ± 0.003 | 0.373 ± 0.017 | 0.150 ± 0.009 | 0.793 ± 0.015 | 0.531 ± 0.012 | 2.201 ± 0.017 | 4.146 ± 0.061 |
| T 144 h | 0.015 ± 0.007 | 0.119 ± 0.007 | 0.045 ± 0.006 | 0.696 ± 0.018 | 0.168 ± 0.018 | 1.936 ± 0.010 | 5.263 ± 0.078 |
| Rhodotorula kratochvilovae + Desmodesmus dimorphus | |||||||
| Time | Lutein | Torularhodin | Betacarotene | Total Carotenoids | Total Chlorophylls | Ergosterol | Ubiquinone |
| T 0 h A+Y | 0.027 ± 0.003 | 0.120 ± 0.003 | 0.129 ± 0.005 | 0.307 ± 0.011 | 0.380 ± 0.024 | 2.343 ± 0.102 | 5.417 ± 0.187 |
| T 24 h | 0.023 ± 0.004 | 0.189 ± 0.007 | 0.135 ± 0.008 | 0.539 ± 0.065 | 0.225 ± 0.019 | 1.106 ± 0.099 | 3.784 ± 0.164 |
| T 48 h | 0.022 ± 0.002 | 1.180 ± 0.045 | 0.119 ± 0.021 | 1.514 ± 0.130 | 0.203 ± 0.034 | 3.315 ± 0.085 | 3.622 ± 0.0155 |
| T 66 h | 0.022 ± 0.003 | 0.585 ± 0.065 | 0.054 ± 0.010 | 0.762 ± 0.087 | 0.215 ± 0.015 | 1.580 ± 0.107 | 2.201 ± 0.087 |
| T 72 h | 0.024 ± 0.007 | 0.720 ± 0.047 | 0.222 ± 0.33 | 1.115 ± 0.106 | 0.244 ± 0.065 | 2.420 ± 0.074 | 4.861 ± 0.174 |
| T 90 h | 0.031 ± 0.006 | 0.500 ± 0.035 | 0.112 ± 0.012 | 1.061 ± 0.067 | 0.202 ± 0.042 | 2.368 ± 0.102 | 2.501 ± 0.080 |
| T 96 h | 0.051 ± 0.010 | 0.984 ± 0.048 | 0.103 ± 0.008 | 1.339 ± 0.099 | 0.307 ± 0.018 | 2.609 ± 0.140 | 2.123 ± 0.103 |
| T 116 h | 0.080 ± 0.012 | 0.542 ± 0.025 | 0.255 ± 0.041 | 0.998 ± 0.084 | 0.197 ± 0.008 | 2.498 ± 0.109 | 2.342 ± 0.090 |
| T 120 h | 0.091 ± 0.013 | 0.760 ± 0.033 | 0.278 ± 0.034 | 1.286 ± 0.108 | 0.182 ± 0.011 | 3.003 ± 0.132 | 4.364 ± 0.105 |
| T 140 h | 0.103 ± 0.015 | 1.881 ± 0.098 | 0.191 ± 0.015 | 2.488 ± 0.132 | 0.172 ± 0.023 | 4.787 ± 0.140 | 4.568 ± 0.166 |
| T 144 h | 0.120 ± 0.020 | 1.111 ± 0.102 | 0.285 ± 0.024 | 1.785 ± 0.102 | 0.161 ± 0.020 | 3.538 ± 0.108 | 4.587 ± 0.129 |
| Rhodotorula kratochvilovae + Desmodesmus acutus | |||||||
| Time | Lutein | Torularhodin | Betacarotene | Total Carotenoids | Total Chlorophylls | Ergosterol | Ubiquinone |
| T 20 h | 0.066 ± 0.010 | 1.165 ± 0.038 | 0.183 ± 0.008 | 1.977 ± 0.102 | 2.108 ± 0.147 | 3.863 ± 0.201 | 2.102 ± 0.102 |
| T 24 h | 0.109 ± 0.011 | 0.170 ± 0.017 | 0.059 ± 0.017 | 0.831 ± 0.064 | 1.516 ± 0.123 | 6.695 ± 0.345 | 5.939 ± 0.207 |
| T 46 h | 0.037 ± 0.006 | 1.420 ± 0.078 | 0.204 ± 0.013 | 1.856 ± 0.111 | 1.471 ± 0.098 | 3.689 ± 0.187 | 3.174 ± 0.190 |
| T 54 h | 0.053 ± 0.007 | 0.970 ± 0.065 | 0.113 ± 0.007 | 1.474 ± 0.107 | 0.931 ± 0.071 | 4.118 ± 0.158 | 4.385 ± 0.183 |
| T 72 h | 0.094 ± 0.018 | 1.085 ± 0.098 | 0.140 ± 0.021 | 1.832 ± 0.098 | 1.091 ± 0.103 | 4.895 ± 0.201 | 3.462 ± 0.146 |
| T 96 h | 0.030 ± 0.006 | 0.874 ± 0.074 | 0.097 ± 0.007 | 1.149 ± 0.079 | 0.445 ± 0.021 | 2.956 ± 0.162 | 3.527 ± 0.078 |
| T 120 h | 0.090 ± 0.007 | 1.190 ± 0.102 | 0.267 ± 0.023 | 1.897 ± 0.105 | 1.165 ± 0.067 | 2.571 ± 0.089 | 4.568 ± 0.128 |
| T 140 h | 0.031 ± 0.013 | 0.922 ± 0.086 | 0.103 ± 0.012 | 1.212 ± 0.67 | 0.469 ± 0.035 | 3.119 ± 0.134 | 3.722 ± 0.136 |
| T 150 h | 0.044 ± 0.008 | 1.093 ± 0.074 | 0.118 ± 0.023 | 1.521 ± 0.101 | 0.420 ± 0.045 | 3.083 ± 0.156 | 6.088 ± 0.201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szotkowski, M.; Holub, J.; Šimanský, S.; Hubačová, K.; Sikorová, P.; Mariničová, V.; Němcová, A.; Márová, I. Bioreactor Co-Cultivation of High Lipid and Carotenoid Producing Yeast Rhodotorula kratochvilovae and Several Microalgae under Stress. Microorganisms 2021, 9, 1160. https://doi.org/10.3390/microorganisms9061160
Szotkowski M, Holub J, Šimanský S, Hubačová K, Sikorová P, Mariničová V, Němcová A, Márová I. Bioreactor Co-Cultivation of High Lipid and Carotenoid Producing Yeast Rhodotorula kratochvilovae and Several Microalgae under Stress. Microorganisms. 2021; 9(6):1160. https://doi.org/10.3390/microorganisms9061160
Chicago/Turabian StyleSzotkowski, Martin, Jiří Holub, Samuel Šimanský, Klára Hubačová, Pavlína Sikorová, Veronika Mariničová, Andrea Němcová, and Ivana Márová. 2021. "Bioreactor Co-Cultivation of High Lipid and Carotenoid Producing Yeast Rhodotorula kratochvilovae and Several Microalgae under Stress" Microorganisms 9, no. 6: 1160. https://doi.org/10.3390/microorganisms9061160
APA StyleSzotkowski, M., Holub, J., Šimanský, S., Hubačová, K., Sikorová, P., Mariničová, V., Němcová, A., & Márová, I. (2021). Bioreactor Co-Cultivation of High Lipid and Carotenoid Producing Yeast Rhodotorula kratochvilovae and Several Microalgae under Stress. Microorganisms, 9(6), 1160. https://doi.org/10.3390/microorganisms9061160