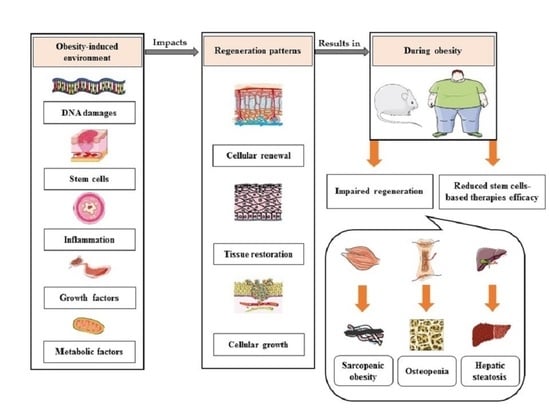
Regeneration during Obesity: An Impaired Homeostasis
Abstract
:Simple Summary
Abstract
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Broken Energy Homeostasis and Obesity Pathogenesis: The Surrounding Concepts. J. Clin. Med. 2018, 7, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanemi, A.; St-Amand, J. Redefining obesity toward classifying as a disease. Eur. J. Intern. Med. 2018, 55, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.-M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tencerova, M.; Figeac, F.; Ditzel, N.; Taipaleenmäki, H.; Nielsen, T.K.; Kassem, M. High-Fat Diet-Induced Obesity Promotes Expansion of Bone Marrow Adipose Tissue and Impairs Skeletal Stem Cell Functions in Mice. J. Bone Miner. Res. 2018, 33, 1154–1165. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.V.; Renovato-Martins, M.; Ribeiro-Pereira, C.; Citelli, M.; Barja-Fidalgo, C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obeity 2016, 24, 2522–2532. [Google Scholar] [CrossRef]
- Ulum, B.; Teker, H.T.; Sarikaya, A.; Balta, G.; Kuşkonmaz, B.; Uckan, D.; Aerts-Kaya, F. Bone marrow mesenchymal stem cell donors with a high body mass index display elevated endoplasmic reticulum stress and are functionally impaired. J. Cell. Physiol. 2018, 233, 8429–8436. [Google Scholar] [CrossRef]
- Pérez, L.M.; Bernal, A.; Martín, N.S.; Gálvez, B.G. Obese-derived ASCs show impaired migration and angiogenesis properties. Arch. Physiol. Biochem. 2013, 119, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-L.; Diekman, B.O.; Jain, D.; Guilak, F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: The effects of free fatty acids. Int. J. Obes. 2012, 37, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Wong, W.T.; Wang, D.H. Obesity reshapes stem cell extracellular vesicles. Cytom. Part A 2018, 93, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Kraakman, M.J.; Murphy, A.J.; Jandeleit-Dahm, K.; Kammoun, H.L. Macrophage Polarization in Obesity and Type 2 Diabetes: Weighing Down Our Understanding of Macrophage Function? Front. Immunol. 2014, 5, 470. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Varga, T.; Mounier, R.; Patsalos, A.; Gogolák, P.; Peloquin, M.; Horvath, A.; Pap, A.; Daniel, B.; Nagy, G.; Pintye, E.; et al. Macrophage PPARγ, a Lipid Activated Transcription Factor Controls the Growth Factor GDF3 and Skeletal Muscle Regeneration. Immunity 2016, 45, 1038–1051. [Google Scholar] [CrossRef] [Green Version]
- Varga, T.; Mounier, R.; Gogolak, P.; Poliska, S.; Chazaud, B.; Nagy, L. Tissue LyC6− Macrophages Are Generated in the Absence of Circulating LyC6− Monocytes and Nur77 in a Model of Muscle Regeneration. J. Immunol. 2013, 191, 5695–5701. [Google Scholar] [CrossRef] [Green Version]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Póliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Zhu, M.; Zhang, S.; Foretz, M.; Viollet, B.; Du, M. Obesity impairs skeletal muscle regeneration via inhibition of AMP-activated protein kinase. Diabetes 2015, 65, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Werner, J.-U.; Milerski, S.; Hamp, C.M.; Kuzenko, T.; Jähnert, M.; Gottmann, P.; De Roy, L.; Warnecke, D.; Abaei, A.; et al. Diet-Induced Obesity Affects Muscle Regeneration After Murine Blunt Muscle Trauma—A Broad Spectrum Analysis. Front. Physiol. 2018, 9, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, A.E.; Daughtry, M.R.; Yen, C.; Kirkpatrick, L.T.; Shi, H.; Gerrard, D.E. Dual effects of obesity on satellite cells and muscle regeneration. Physiol. Rep. 2020, 8, e14511. [Google Scholar] [CrossRef] [PubMed]
- Dungan, C.M.; Peck, B.D.; Walton, R.G.; Huang, Z.; Bamman, M.M.; Kern, P.A.; Peterson, C.A. In vivo analysis of γH2AX+ cells in skeletal muscle from aged and obese humans. FASEB J. 2020, 34, 7018–7035. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Qu, H.; Zheng, Y.; Liao, Q.; Zhang, L.; Liao, X.; Xiong, X.; Wang, Y.; Zhang, R.; Wang, H.; et al. Mitochondrial glycerol 3-phosphate dehydrogenase promotes skeletal muscle regeneration. EMBO Mol. Med. 2018, 10, e9390. [Google Scholar] [CrossRef] [PubMed]
- Duguez, S.; Féasson, L.; Denis, C.; Freyssenet, D. Mitochondrial biogenesis during skeletal muscle regeneration. Am. J. Physiol. Metab. 2002, 282, E802–E809. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, A.; Kotake, N.; Yamada, S. Muscle regeneration occurs to coincide with mitochondrial biogenesis. Mol. Cell. Biochem. 2010, 349, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, A.; Kotake, N.; Kawachi, T.; Shiozuka, M.; Yamada, S.; Matsuda, R. Mitochondrial adaptations in skeletal muscle to hindlimb unloading. Mol. Cell. Biochem. 2010, 350, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A. Invited Review: Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001, 90, 1137–1157. [Google Scholar] [CrossRef]
- Hood, D.A.; Irrcher, I.; Ljubicic, V.; Joseph, A.-M. Coordination of metabolic plasticity in skeletal muscle. J. Exp. Biol. 2006, 209, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.F.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; E Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Metab. 2015, 309, E1019–E1031. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, R.A.; Sheedfar, F.; Morrison, M.C.; Hommelberg, P.P.H.; Kor, D.; Kloosterhuis, N.J.; Gruben, N.; A Youssef, S.; De Bruin, A.; Hofker, M.H.; et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 2015, 7, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Potter, V.J.; Valentine, R.J.; Wilund, K.R.; Antao, N.; Baynard, T.; Woods, J.A. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am. J. Physiol. Metab. 2009, 296, E1164–E1171. [Google Scholar] [CrossRef] [Green Version]
- Perandini, L.A.; Chimin, P.; Lutkemeyer, D.D.S.; Câmara, N.O.S. Chronic inflammation in skeletal muscle impairs satellite cells function during regeneration: Can physical exercise restore the satellite cell niche? FEBS J. 2018, 285, 1973–1984. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Hong, S.-H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [Green Version]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef] [PubMed]
- Khoo, N.K.; Fazzari, M.; Chartoumpekis, D.V.; Li, L.; Guimaraes, D.A.; Arteel, G.E.; Shiva, S.; Freeman, B.A. Electrophilic nitro-oleic acid reverses obesity-induced hepatic steatosis. Redox Biol. 2019, 22, 101132. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Gilgenkrantz, H. The impact of steatosis on liver regeneration. Horm. Mol. Biol. Clin. Investig. 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Berglund, E.D.; Yu, X.; Wang, M.-Y.; Evans, M.R.; Scherer, P.E.; Holland, W.L.; Charron, M.J.; Roth, M.G.; Unger, R.H. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13217–13222. [Google Scholar] [CrossRef] [Green Version]
- Sáinz, N.; Barrenetxe, J.; Moreno-Aliaga, M.J.; Martínez, J.A. Leptin resistance and diet-induced obesity: Central and peripheral actions of leptin. Metabolism 2015, 64, 35–46. [Google Scholar] [CrossRef]
- Raut, P.K.; Kim, S.-H.; Choi, D.Y.; Jeong, G.-S.; Park, P.-H. Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: Critical roles of estrogen receptor signaling and reactive oxygen species production. Biochem. Pharmacol. 2019, 161, 73–88. [Google Scholar] [CrossRef]
- Barrios-Correa, A.A.; Estrada, J.A.; Contreras, I. Leptin Signaling in the Control of Metabolism and Appetite: Lessons from Animal Models. J. Mol. Neurosci. 2018, 66, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef]
- Laron, Z.; Werner, H. Insulin: A Growth Hormone and Potential Oncogene. Pediatr. Endocrinol. Rev. 2020, 17, 191–197. [Google Scholar] [PubMed]
- Tencerova, M.; Okla, M.; Kassem, M. Insulin Signaling in Bone Marrow Adipocytes. Curr. Osteoporos. Rep. 2019, 17, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietto, J.; Tencerova, M.; Frost, M.; Figeac, F.; Nielsen, T.K.; Ali, D.; Lauterlein, J.L.; Andersen, T.L.; Haakonsson, A.K.; Rauch, A.; et al. Faculty Opinions recommendation of Obesity-Associated Hypermetabolism and Accelerated Senescence of Bone Marrow Stromal Stem Cells Suggest a Potential Mechanism for Bone Fragility. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2020, 27, 2050–2062. [Google Scholar] [CrossRef]
- Cornish, J.; Wang, T.; Lin, J.-M. Role of Marrow Adipocytes in Regulation of Energy Metabolism and Bone Homeostasis. Curr. Osteoporos. Rep. 2018, 16, 116–122. [Google Scholar] [CrossRef]
- Muruganandan, S.; Govindarajan, R.; Sinal, C.J.; Shanmugam, M.; Rajgopal, G. Bone Marrow Adipose Tissue and Skeletal Health. Curr. Osteoporos. Rep. 2018, 16, 434–442. [Google Scholar] [CrossRef]
- Röszer, T.; Józsa, T.; Kiss-Tóth, E.D.; De Clerck, N.; Balogh, L. Leptin receptor deficient diabetic (db/db) mice are compromised in postnatal bone regeneration. Cell Tissue Res. 2013, 356, 195–206. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Li, H.-M.; Ye, Z.-H. Microenvironment of liver regeneration in liver cancer. Chin. J. Integr. Med. 2017, 23, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Boilly, B.; Faulkner, S.; Jobling, P.; Hondermarck, H. Nerve Dependence: From Regeneration to Cancer. Cancer Cell 2017, 31, 342–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Lv, T.-R.; Zhou, J.-C.; Qin, X.-D. Effects of obesity on the healing of bone fracture in mice. J. Orthop. Surg. Res. 2018, 13, 145. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Harrison, F.E.; Xia, F. Altered DNA repair; an early pathogenic pathway in Alzheimer’s disease and obesity. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [Green Version]
- Mazon, J.N.; De Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The impact of obesity on neurodegenerative diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Payab, M.; Goodarzi, P.; Heravani, N.F.; Hadavandkhani, M.; Zarei, Z.; Falahzadeh, K.; Larijani, B.; Rahim, F.; Arjmand, B. Stem Cell and Obesity: Current State and Future Perspective. Atherosclerosis 2018, 1089, 1–22. [Google Scholar] [CrossRef]
- Matsushita, K.; Dzau, V.J. Mesenchymal stem cells in obesity: Insights for translational applications. Lab. Investig. 2017, 97, 1158–1166. [Google Scholar] [CrossRef]
- Ghanemi, A.; Melouane, A.; Mucunguzi, O.; Yoshioka, M.; St-Amand, J. Energy and metabolic pathways in trefoil factor family member 2 (Tff2) KO mice beyond the protection from high-fat diet-induced obesity. Life Sci. 2018, 215, 190–197. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Kim, M.W.; Khan, A.; Jo, M.H.; Rehman, S.U.; Khan, M.S.; Bin Abid, N.; Khan, M.; Ullah, R.; et al. Adiponectin homolog novel osmotin protects obesity/diabetes-induced NAFLD by upregulating AdipoRs/PPARα signaling in ob/ob and db/db transgenic mouse models. Metabolism 2019, 90, 31–43. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanemi, A.; Yoshioka, M.; St-Amand, J. Regeneration during Obesity: An Impaired Homeostasis. Animals 2020, 10, 2344. https://doi.org/10.3390/ani10122344
Ghanemi A, Yoshioka M, St-Amand J. Regeneration during Obesity: An Impaired Homeostasis. Animals. 2020; 10(12):2344. https://doi.org/10.3390/ani10122344
Chicago/Turabian StyleGhanemi, Abdelaziz, Mayumi Yoshioka, and Jonny St-Amand. 2020. "Regeneration during Obesity: An Impaired Homeostasis" Animals 10, no. 12: 2344. https://doi.org/10.3390/ani10122344
APA StyleGhanemi, A., Yoshioka, M., & St-Amand, J. (2020). Regeneration during Obesity: An Impaired Homeostasis. Animals, 10(12), 2344. https://doi.org/10.3390/ani10122344