A Morphological and Morphometric Dental Analysis as a Forensic Tool to Identify the Iberian Wolf (Canis Lupus Signatus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphometric Study
2.2. Morphological Description
2.3. Statistical Analysis
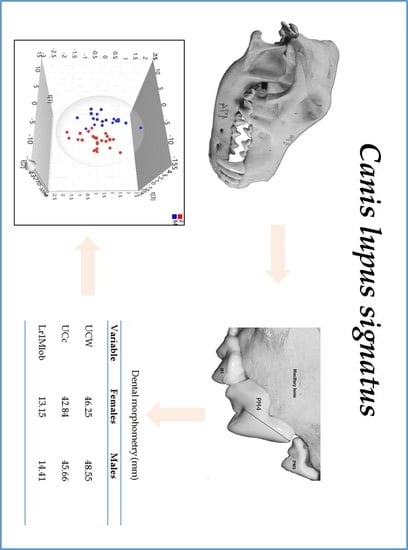
3. Results
3.1. Dental Morphology
3.1.1. The Teeth of the Iberian Wolf
Incisors
Canines
Premolars
Molar Teeth
3.1.2. Normal Occlusion (the Closing of the Jaw)
3.2. Morphometric Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Souviron, R. Forensic odontology. In Forensic Pathology: Principles and Practice, 1st ed.; Dolinak, D., Matshes, E., Lew, E., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 605–629. [Google Scholar]
- Murmann, D.C.; Brumit, P.C.; Schrader, B.A.; Senn, D.R. A comparison of animal jaws and bite mark patterns. J. Forensic Sci. 2006, 51, 846–860. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.W.; Loveridge, A.J. Biology and Conservation of Wild Felids; Oxford University Press Inc.: New York, NY, USA, 2010. [Google Scholar]
- Moraitis, K.; Spiliopoulou, C. Forensic implications of carnivore scavenging on human remains recovered from outdoor locations in Greece. J. Forensic Leg. Med. 2010, 17, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Haglund, W.D. Dogs and coyotes: Postmortem involvement with human remains. In Forensic Taphonomy: The Postmortem Fate of Human Remains; Haglund, W.D., Sorg, M.H., Eds.; CRC Press LLC: Boca Raton, FL, USA, 1997; pp. 386–400. [Google Scholar]
- Morton, R.; Lord, W. Taphonomy of child-sized remains: A study of scattering and scavenging in Virginia, USA. J. Forensic Sci. 2006, 51, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Santoro, V.; Smaldone, G.; Lozito, P.; Smaldone, M.; Introna, F. A forensic approach to fatal dog attacks. A case study and review of the literature. Forensic Sci. Int. 2011, 206, e37–e42. [Google Scholar] [CrossRef]
- Pimenta, V.; Barroso, I.; Boitani, L.; Beja, P. Wolf predation on cattle in Portugal: Assessing the effects of husbandry systems. Biol. Conserv. 2017, 207, 17–26. [Google Scholar] [CrossRef]
- González, J.A.; Carvalho, A.M.; Vallejo, J.R.; Amich, F. Plant-based remedies for wolf bites and rituals against wolves in the Iberian Peninsula: Therapeutic opportunities and cultural values for the conservation of biocultural diversity. J. Ethnopharmacol. 2017, 209, 124–139. [Google Scholar] [CrossRef]
- Garff, E.L.; Mesli, V.; Delannoy, Y.; Pollard, J.; Becart, A.; Hedouin, V. Domestic predation of an elder: A fatal dog attack case. J. Forensic Sci. 2017, 62, 1379–1382. [Google Scholar] [CrossRef]
- Cozza, K.; Fico, R.; Battistini, M.L.; Rogers, E. The damage-conservation interface illustrated by predation on domestic livestock in central Italy. Biol. Conserv. 1996, 78, 329–336. [Google Scholar] [CrossRef]
- Steffens, K.; Sanders, M.; Gleeson, D.; Pullen, K.; Stowe, C. Identification of predators at black-fronted tern Chlidonias albostriatus nests, using mtDNA analysis and digital video recorders. N. Z. J. Ecol. 2012, 36, 48–55. [Google Scholar]
- Gidna, A.; Yravedra, J.; Domínguez-Rodrigo, M. A cautionary note on the use of captive carnivores to model wild predator behavior: A comparison of bone modification patterns on long bones by captive and wild lions. J. Archaeol. Sci. 2013, 40, 1903–1910. [Google Scholar] [CrossRef]
- Young, A.; Stillman, R.; Smith, M.J.; Korstjens, A.H. An experimental study of vertebrate scavenging behavior in a northwest European woodland context. J. Forensic Sci. 2014, 59, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Severtsov, A.S.; Kormylitsin, A.A.; Severtsova, E.A.; Yatsuk, I.A. Functional differentiation of teeth in the wolf (Canis lupus, Canidae, Carnivora). Biol. Bull. Russ. Acad. Sci. 2016, 43, 1271–1280. [Google Scholar] [CrossRef]
- Geiger, M.; Gendron, K.; Willmitzer, F.; Sánchez-Villagra, M. Unaltered sequence of dental, skeletal, and sexual maturity in domestic dogs compared to the wolf. Zool. Lett. 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmons, M.; Beebe, D. Oral anatomy and physiology. In Wigg‘s Veterinary Dentistry. Principles and Practice, 2nd ed.; Lobprise, H., Dodd, J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2019; pp. 1–15. [Google Scholar]
- Schaller, O. Nomenclatura Anatómica Veterinaria Ilustrada, 1st ed.; Acribia, S.A.: Zaragoza, Spain, 1996. [Google Scholar]
- Morales, A.D.; Osorio, A.C.; Fernández, R.F.; Dennes, E.L. Desarrollo de un modelo SIMCA para la clasificación de kerosinas mediante el empleo de la espectroscopía infrarroja. Química Nova. 2008, 31, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, J.J.; Georgevsky, D.; Valenzuela, M.; McGreevy, P.D. A pilot study of sexual dimorphism in the head morphology of domestic dogs. J. Vet. Behav. 2014, 9, 43–46. [Google Scholar] [CrossRef]
- Vila, C.; Savolainen, P.; Maldonado, J.E.; Amorim, I.R.; Rice, J.E.; Honeycutt, R.L.; Crandall, K.A.; Lundeberg, J.; Wayne, R.K. Multiple and ancient origins of the domestic dog. Science 1997, 276, 1687–1689. [Google Scholar] [CrossRef]
- Yravedra, J.; García-Vargas, E.; Maté-González, M.A.; Aramendi, J.; Palomeque-González, J.F.; Vallés-Iriso, J.; Matesanz-Vicente, J.; González-Aguilera, D.; Domínguez-Rodrigo, M. The use of micro-photogrammetry and geometric morphometrics for identifying carnivore agency in bone assemblages. J. Archaeol. Sci. Rep. 2017, 14, 106–115. [Google Scholar] [CrossRef]
- Yravedra, J.; Lagos, L.; Bárcena, F. A taphonomic study of wild wolf (Canis lupus). Modification of horse bones in northwestern Spain. J. Taphon. 2011, 9, 37–65. [Google Scholar]
- Aramendi, J.; Maté-González, M.A.; Yravedra, J.; Ortega, M.C.; Arriaza, M.C.; González-Aguilera, D.; Baquedano, E.; Domínguez-Rodrigo, M. Discerning carnivore agency through the three-dimensional study of tooth pits: Revisiting crocodile feeding behaviour at FLK- Zinj and FLK NN3 (Olduvai Gorge, Tanzania). Paleoecol. Geogr. Palaeoclimatol. Palaeoecol. 2017, 488, 93–102. [Google Scholar] [CrossRef]
- Iglesias, A.; España, A.J.; España, J. Lobos Ibéricos. Anatomía, Ecología Y Conservación, 1st ed.; Náyade Nature: Valladolid, Spain, 2017; p. 532. [Google Scholar]
- Haynes, G. Evidence of carnivore gnawing on Pleistocene and recent mammalian bones. Paleobiology 1980, 6, 341–351. [Google Scholar] [CrossRef]
- Binford, L.R. Bones: Ancient Men and Modern Myths (Studies in Archeology), 1st ed.; Academic Press: London, UK, 1981. [Google Scholar]
- Haynes, G. A guide for differentiating mammalian carnivore taxa responsible for gnaw damage to herbivore limb bones. Paleobiology 1983, 9, 164–172. [Google Scholar] [CrossRef]
- Fosse, P.; Wajrak, A.; Fourvel, J.B.; Madelaine, S.; Esteban-Nadal, M.; Cáceres, I.; Yravedra, J.; Brugal, J.P.; Prucca, A.; Haynes, G. Bone modification by modern wolf (Canis lupus): A taphonomic study from their natural feeding places. J. Taphon. 2012, 10, 197–217. [Google Scholar]
- Parkinson, J.; Plummer, T.; Bose, R. A Gis-based approach to documenting large canid damage to bones. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 409, 57–71. [Google Scholar] [CrossRef]
- Sala, N.; Arsuaga, J.L.; Haynes, G. Taphonomic comparison of bone modifications caused by wild and captive wolves (Canis lupus). Quat. Int. 2014, 330, 126–135. [Google Scholar] [CrossRef]
- Andres, M.; Gidna, A.; Yravedra, J.; Dominguez-Rodrigo, M. A study of dimensional differences of tooth marks (pits and scores) on bones modified by small and large carnivores. Archaeol. Anthropol. Sci. 2012, 4, 209–219. [Google Scholar] [CrossRef]
- Yravedra, J.; Andrés, M.; Domínguez-Rodrigo, M. A taphonomic study of the African wild dog (Lycaon pictus). Archaeol. Anthropol. Sci. 2014, 6, 113–124. [Google Scholar] [CrossRef]
- Yravedra, J.; Maté-González, M.Á.; Courtenay, L.A.; González-Aguilera, D.; Fernández, M.F. The use of canid tooth marks on bone for the identification of livestock predation. Sci. Rep. 2019, 9, 16301. [Google Scholar] [CrossRef] [Green Version]
- Drake, A.G.; Klingenberg, C.P. Large-scale diversification of skull shape in domestic dogs: Disparity and modularity. Am. Nat. 2010, 175, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Ameen, C.; Hulme-Beaman, A.; Evin, A.; Germonpré, M.; Britton, K.; Cucchi, T.; Larson, G.; Dobney, K. A landmark-based approach for assessing the reliability of mandibular tooth crowding as a marker of dog domestication. J. Archaeol. Sci. 2017, 85, 41–50. [Google Scholar] [CrossRef]
- Germonpré, M.; Sablin, M.V.; Stevens, R.E.; Hedges, R.E.M.; Hofreiter, M.; Stiller, M.; Després, V.R. Fossil dogs and wolves from Paleolithic sites in Belgium, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes. J. Archaeol. Sci. 2009, 36, 473–490. [Google Scholar] [CrossRef]
- Blanco, J.C. Lobo-Canis lupus Linnaeus, 1758. In Enciclopedia Virtual de los Vertebrados Españoles. Sociedad de Amigos del MNCN – MNCN – CSIC; Salvador, A., Barja, I., Eds.; Museo Nacional de Ciencias Naturales CSIC: Madrid, Spain, 2017; pp. 1–25. [Google Scholar]
- Toledo, G.V.; Ortega, O.F.; Fonseca, G.M.; García-Ruíz, C.; Pérez-LLoret, P. Morphometric analysis of bite mark patterns caused by domestic dogs (Canis lupus familiaris) using dental wax registers. Int. J. Morphol. 2019, 37, 885–893. [Google Scholar] [CrossRef]
- Guitián-Rivera, J.; Sánchez-Canals, J.L.; de Castro, A.; Bas López, S. Nota sobre dimorfismo sexual en algunos cráneos de lobo (Canis lupus L) de Galicia. In Trabajos Compostelanos de Biología 8; Universidad de Santiago de Compostela: La Coruña, Spain, 1979; pp. 87–94. [Google Scholar]
- Valverde, J.; Hidalgo, A. Sobre el lobo (Canis lupus) ibérico: I. Dimorfismo sexual en cráneos. Doñana Acta Vert 1974, 1, 233–244. [Google Scholar]
- Machado, M.G. Cánidos Ibéricos: Evaluación de la Morfología Cefálica Con Métodos Clásicos y Actuales de Diagnóstico Por Imagen del lobo Ibérico, Canis Lupus Signatus; Universidad de León España: León, Spain, 2004. [Google Scholar]
- Curth, S.; Fischer, M.S.; Kupczik, K. Patterns of integration in the canine skull: An inside view into the relationship of the skull modules of domestic dogs and wolves. Zoology (Jena, Germany) 2017, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Toledo, G.V.; Ibarra, M.L.; Rojas, E.V.; Ciocca, G.L.; Rocha, D.N.; Jara, V.G. Estudio preliminar de patrones de mordedura según forma del cráneo, mediante el análisis morfológico y morfométrico de semiarcadas dentarias de perro doméstico (Canis familiaris) con fines de identificación. Int. J. Morphol. 2012, 30, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Sweet, D.; Pretty, I.A. A look at forensic dentistry-part 2: Teeth as weapons of violence-identification of bitemark perpetrators. Br. Dent. J. 2001, 190, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Long, K. Wolves. A Wildlife Handbook; Johnson Books: Boulder, CO, USA, 1996. [Google Scholar]
- Doring, S.; Arzi, B.; Winer, J.N.; Kass, P.H.; Verstraete, F.J.M. Dental and temporomandibular joint pathology of the grey wolf (Canis lupus). J. Comp. Pathol. 2018, 160, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Gipson, P.; Ballard, W.; Nowak, R.; Mech, L. Accuracy and precision of estimating age of gray wolves by tooth wear. J. Wildl. Manag. 2000, 64, 752–758. [Google Scholar] [CrossRef]
- Kieser, J.A.; Groeneveld, H.T. Mandibulodental allometry in the African wild dog, Lycaon pictus. J. Anat. 1992, 181 Pt 1, 133–137. [Google Scholar]
- Ralls, K. Mammals in which females are larger than males. Q. Rev. Biol. 1976, 51, 245–276. [Google Scholar] [CrossRef]
- Reiss, M. Males bigger, females biggest. New Sci. 1982, 96, 226–229. [Google Scholar]
- Frayer, D.; Wolpoff, M. Sexual dimorphism. Annu. Rev. Anthropol. 2003, 14, 429–473. [Google Scholar] [CrossRef]
- Stockard, C.R.; Anderson, O.D.; James, W.T.; Wistar Institute of Anatomy and Biology. The Genetic and Endocrinic Basis for Differences in form and Behavior: As Elucidated by Studies of Contrasted Pure-Line Dog Breeds and Their Hybrids, 1st ed.; The Wistar Institute of Anatomy and Biology: Philadelphia, PA, USA, 1941. [Google Scholar]








| Variable | Acronym | Variable | Acronym |
|---|---|---|---|
| Maximum maxillary (upper) intercanine width | UCW * | Maximum mandibular (lower) intercanines width | LCW |
| Maxillary distance between the cusp tip of the canine teeth | UCc * | Mandibular distance between the cusp tip of the canine teeth | LCc |
| Maxillary distance between the palatal surfaces of the canine teeth | UbC * | Mandibular distance between the lingual surfaces of the canine teeth | LbC |
| Maximum maxillary inter-third incisor teeth width | UiW * | Maxillary distance between the cusp tip of the third incisor teeth | Uic * |
| Maxillary distance between the palatal surfaces of the third incisor teeth | Ubi * | Maxillary distance between the cusp tip of the 1st premolar teeth | U1PMc |
| Mandibular distance between the cusp tip of the 1st premolar teeth | L1PMc | Maxillary distance between the cusp tip of the 4th premolar teeth | U4PMc * |
| Mandibular distance between the cusp tip of the 1st molar teeth | L1Mc | Distance between a line drawn behind the third maxillary incisors and a point located between both central incisor teeth on the rostral face of incisive bone | UPi * |
| Distance between a line drawn behind of third maxillary canines and a point located between both central incisor teeth on the rostral face of incisive bone | UPC * | Distance between a line drawn behind maxillary 1st premolars and a point located between both central incisor teeth on the rostral face of the incisive bone | UP1PM * |
| Right maxillary distance between the cusp tip of the 1st premolar–canine teeth | Ur1PMCc | Left maxillary distance between the cusp tip of the 1st premolar–canine teeth | Ul1PMCc * |
| Right mandibular distance between the cusp tip of the 1st premolar–canine teeth | Lr1PMCc | Left mandibular distance between the cusp tip of the 1st premolar–canine teeth | Ll1PMCc |
| Right maxillary distance between the cusp tip of the 1st–2nd premolar teeth | Ur1-2PMc * | Left maxillary distance between the cusp tip of the 1st–2nd premolar teeth | Ul1-2PMc |
| Right mandibular distance between the cusp tip of the 1st–2nd premolar teeth | Lr1-2PMc | Left mandibular distance between the cusp tip of the 1st–2nd premolar teeth | Ll1-2PMc |
| Width of the right maxillary canine teeth | UrCWd | Length of the right maxillary canine teeth | UrCLe |
| Width of the left maxillary canine teeth | UlCWd * | Length of the left maxillary canine teeth | UlCLe * |
| Width of the right mandibular canine teeth | LrCWd | Length of the right mandibular canine teeth | LrCLe |
| Width of the left mandibular canine teeth | LlCWd | Length of the left mandibular canine teeth | LlCLe |
| Length of the right maxillary 4th premolar mesial tubercle | Ur4PMtub * | Length of the left maxillary 4th premolar mesial tubercle | Ul4PMtub |
| Right mandibular 1st molar tubercle length(includes intermediate and mesial tubercles) | Lr1Mtub * | Length of the left mandibular 1st molar tubercle(including the intermediate and mesial tubercles) | Ll1Mtub |
| Va | Lv (mm) | Mean | Va | Lv (mm) | Mean |
|---|---|---|---|---|---|
| UCW | F < 46.25 | 44.7 | Ur1PMCc | F < 13.76 | 16.48 |
| M > 48.55 | 48.4 | M > 20.13 | 18.4 | ||
| LCW | F < 39.15 | 38.66 | Ul1PMCc | F < 15.27 | 16.6 |
| M > 24.23 | 41.91 | M > 20.13 | 18.39 | ||
| UCc | F < 42.84 | 41.27 | UrCWd | F < 7.87 | 8.28 |
| M > 45.66 | 45.01 | M > 9.36 | 9.23 | ||
| LCc | F < 37.06 | 36.91 | UrCLe | F < 12.34 | 12.62 |
| M > 41.07 | 40.09 | M > 13.62 | 14.2 | ||
| UbC | F < 24.65 | 25.39 | UlCWd | F < 7.88 | 8.13 |
| M > 28.04 | 29.14 | M > 9.37 | 9.11 | ||
| LbC | F < 13.06 | 12.91 | UlCLe | F < 12.36 | 12.82 |
| M > 14.87 | 14.71 | M > 14.05 | 13.99 | ||
| UiW | F < 33.10 | 32.04 | LrCLe | F < 12.44 | 12.7 |
| M > 34.16 | 34.18 | M > 14.36 | 14.27 | ||
| Uic | F < 29.81 | 30.46 | LlCLe | F < 13 | 12.48 |
| M > 33.39 | 31.84 | M > 13.69 | M: 14.02 | ||
| Ubi | F < 16.47 | 16.38 | Ur4PMtub | F/M < 17.43 | F: 16.41 |
| M > 18.61 | 17.68 | M > 17.43 | 17.02 | ||
| U1PMc | F < 31.83 | 32.64 | Ul4PMtub | F/M < 7.43 | F: 16.32 |
| M > 36.71 | 34.94 | M > 7.43 | 17 | ||
| U4PMc | F < 60.55 | 58.59 | Lr1Mtub | F < 3.15 | 13.06 |
| M > 64.08 | 63.57 | M > 4.41 | 14.04 | ||
| L1Mc | F/M < 54.11 | F: 50.54 | Ll1Mtub | F < 13.10 | 13.09 |
| M > 54.11 | 54.28 | M > 14.4 | 14.02 | ||
| UP1PM | F < 43.35 | 42.92 | |||
| M > 45.84 | 46.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo González, V.; Ortega Ojeda, F.; Fonseca, G.M.; García-Ruiz, C.; Navarro Cáceres, P.; Pérez-Lloret, P.; Marín García, M.d.P. A Morphological and Morphometric Dental Analysis as a Forensic Tool to Identify the Iberian Wolf (Canis Lupus Signatus). Animals 2020, 10, 975. https://doi.org/10.3390/ani10060975
Toledo González V, Ortega Ojeda F, Fonseca GM, García-Ruiz C, Navarro Cáceres P, Pérez-Lloret P, Marín García MdP. A Morphological and Morphometric Dental Analysis as a Forensic Tool to Identify the Iberian Wolf (Canis Lupus Signatus). Animals. 2020; 10(6):975. https://doi.org/10.3390/ani10060975
Chicago/Turabian StyleToledo González, Víctor, Fernando Ortega Ojeda, Gabriel M. Fonseca, Carmen García-Ruiz, Pablo Navarro Cáceres, Pilar Pérez-Lloret, and María del Pilar Marín García. 2020. "A Morphological and Morphometric Dental Analysis as a Forensic Tool to Identify the Iberian Wolf (Canis Lupus Signatus)" Animals 10, no. 6: 975. https://doi.org/10.3390/ani10060975
APA StyleToledo González, V., Ortega Ojeda, F., Fonseca, G. M., García-Ruiz, C., Navarro Cáceres, P., Pérez-Lloret, P., & Marín García, M. d. P. (2020). A Morphological and Morphometric Dental Analysis as a Forensic Tool to Identify the Iberian Wolf (Canis Lupus Signatus). Animals, 10(6), 975. https://doi.org/10.3390/ani10060975