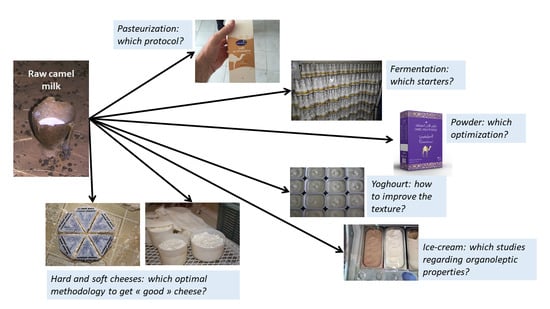
Recent Advances in Camel Milk Processing
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pasteurized Milk
2.1. Current Global Conditions for Pasteurization of Camel Milk
2.2. Indicators of Camel Milk Pasteurization
2.3. Impact of Pasteurization on Physical Properties of Camel Milk
2.4. Camel Milk Protein Behavior Following Heat Treatment
2.5. Sterilized Milk
2.6. Antimicrobial Activity and Pasteurization of Camel Milk
3. Fermented Milk
3.1. Microflora of Raw Camel Milk
3.1.1. Nonpathogenic Microflora in Raw Milk
3.1.2. Pathogenic Microflora of Raw Camel Milk
3.2. Diversity of Fermented Camel Milk
4. Camel Cheese
4.1. The Challenge of Coagulation
4.2. Rheological and Microstructural Studies
4.3. Comparative “Behavior” with Cow Cheese
4.4. The Challenge of Industrial Development
5. Camel Milk Powder
5.1. The Drying Technologies
5.2. Interests and Limitations of the Technologies Used for Spraying Camel Milk
5.3. The Challenge for Camel Milk Powder Development
6. Other Products
6.1. Yogurt
6.2. Butter and Sweet
6.3. Non-Alimentary Processing of Camel-Milk
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marsh, A.J.; Hill, C.; Paul Ross, R.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Faye, B. The camel: New challenges for a sustainable development. Trop. Anim. Health Prod. 2016, 48, 689–692. [Google Scholar] [CrossRef] [Green Version]
- Al-haj, O.A.; Kanhal, H.A. Compositional, technological and nutritional aspects of dromedary camel milk. Int. Dairy J. 2010, 20, 811–821. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B. New Opportunities in the Camel Milk Valorization. In Proceedings of the 5th Conference ISOCARD Recent Advances in Camelids Biology, Health and Production, Laâyoune, Morocco, 12–15 November 2018; Sghiri, A., Kichou, F., Eds.; Pub. IAV Hassan II: Rabat, Morocco, 2018; pp. 45–50. [Google Scholar]
- Abdelgader, A.G.; Alhaider, A.A. The unique medicinal properties of camel products: A review of the scientific evidence. J. Taibah Univ. Med Sci. 2016, 11, 98e103. [Google Scholar] [CrossRef] [Green Version]
- FAOstat. 2021. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 23 March 2021).
- Zhang, B.Y.; Xu, S.; Villalobos-Santeli, J.A.; Huang, J.Y. Fouling characterization of camel milk with comparison to bovine milk. J. Food Eng. 2020, 285, 11008. [Google Scholar] [CrossRef]
- Lajnaf, R.; Zouari, A.; Trigui, I.; Attia, H.; Ayadi, M.A. Effect of different heating temperatures on foaming properties of camel milk proteins: A comparison with bovine milk proteins, Int. Dairy J. 2020, 104, 104643. [Google Scholar] [CrossRef]
- Li, R.R.; Yue, H.T.; Shi, Z.Y.; Shen, T.; Yao, H.B.; Zhang, J.W.; Gao, Y.; Yang, J. Protein profile of whole camel milk resulting from commercial thermal treatment. LWT 2020, 134, 110256. [Google Scholar] [CrossRef]
- Yehia, H.M.; Al-Masoud, A.H.; Alarjani, K.M.; Alamri, M.S. Prevalence of methicillin-resistant (mecA gene) and heat-resistant Staphylococcus aureus strains in pasteurized camel milk, J. Dairy Sci. 2020, 103, 5947–5963. [Google Scholar] [CrossRef]
- Bragason, E.; Berhe, T.; Dashe, D.; Sørensen, K.I.; Eshetu Guya, M.; Hansen, E.B. Antimicrobial activity of novel Lactococcus lactis strains against Salmonella Typhimurium DT12, Escherichia coli O157:H7 VT− and Klebsiella pneumoniae in raw and pasteurised camel milk. Int. Dairy J. 2020, 111, 104832. [Google Scholar] [CrossRef]
- Gammoh, S.; Alu’datt, M.H.; Tranchant, C.C.; Al-U’datt, D.G.; Alhamad, M.N.; Rababah, T.; Kubow, S.; Haddadin, M.S.Y.; Ammari, Z.; Maghaydah, S.; et al. Modification of the functional and bioactive properties of camel milk casein and whey proteins by ultrasonication and fermentation with Lactobacillus delbrueckii subsp. Lactis. LWT 2020, 129, 109501. [Google Scholar] [CrossRef]
- Sobti, B.; Aljneibi, A.H.A.; Seraidy, H.A.A.; Alnaqbi, A.A.H.; Al Zain, B.; Ramachandran, T.; Hamed, F.; Kamal-Eldin, A. Short communication: The effect of pectin and sodium alginate on labans made from camel milk and bovine milk. J. Dairy Sci. 2021. [CrossRef]
- Mortazavi, S.M.; Jalali, H.; Hamidreza Ziaolhagh, S. Production of a probiotic camel milk enriched with pomegranate peel powder. Iran. Food Sci. Technol. Res. J. 2021, 16, 123–132. [Google Scholar]
- Zhadyra, S.; Han, X.; Anapiyayev, B.B.; Tao, F.; Xu, P. Bacterial diversity analysis in Kazakh fermented milks Shubat and Ayran by combining culture-dependent and culture-independent methods. LWT 2021, 141, 110877. [Google Scholar] [CrossRef]
- Sharma, A.; Lavania, M.; Singh, R.; Lal, B. Identification and probiotic potential of lactic acid bacteria from camel milk. Saudi J. Biol. Sci. 2021, 28, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef]
- Soleymanzadeh, N.; Mirdamadi, S.; Mirzaei, M.; Kianirad, M. Novel β-casein derived antioxidant and ACE-inhibitory active peptide from camel milk fermented by Leuconostoc lactis PTCC1899: Identification and molecular docking. Int. Dairy J. 2019, 97, 201–208. [Google Scholar] [CrossRef]
- Edalati, E.; Saneei, B.; Alizadeh, M.; Hosseini, S.S.; Zahedi Bialvaei, A.; Taheri, K. Isolation of probiotic bacteria from raw camel’s milk and their antagonistic effects on two bacteria causing food poisoning. New Microbes New Infect. 2019, 27, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mbye, M.; Sobti, B.; Al Nuami, M.K.; Al Shamsi, Y.; Al Khateri, L.; Al Saedi, R.; Saeed, M.; Ramachandran, T.; Hamed, F.; Kamal-Eldin, A. Physicochemical properties, sensory quality, and coagulation behavior of camel versus bovine milk soft unripened cheeses. NFS J. 2020, 20, 28–36. [Google Scholar] [CrossRef]
- Belkheir, B.; Zadi-Karam, H.; Karam, N.E.; Carballo, J.; Centeno, J.A. Effects of selected mesophilic Lactobacillus strains obtained from camel milk on the volatile and sensory profiles of a model short-ripened pressed cows’ milk cheese. Int. Dairy J. 2020, 109, 104738. [Google Scholar] [CrossRef]
- El Hatmi, H.; Jrad, Z.; Mkadem, W.; Chahbani, A.; Oussaief, O.; Ben Zid, M.; Nouha, M.; Zaidi, S.; Khorchani, S.; Belguith, K.; et al. Fortification of soft cheese made from ultrafiltered dromedary milk with Allium roseum powder: Effects on textural, radical scavenging, phenolic profile and sensory characteristics. LWT 2020, 132, 109885. [Google Scholar] [CrossRef]
- Perusko, M.; Ghnimi, S.; Simovic, A.; Stevanovic, N.; Radomirovic, M.; Gharsallaoui, A.; Smiljanic, K.; Van Haute, S.; Stanic-Vucinic, D.; Velickovic, T.C. Maillard reaction products formation and antioxidative power of spray dried camel milk powders increases with the inlet temperature of drying. LWT 2021, 143, 111091. [Google Scholar] [CrossRef]
- Ho, T.M.; Chan, S.; Yago, A.J.E.; Shravya, R.; Bhandari, B.R.; Bansal, N. Changes in physicochemical properties of spray-dried camel milk powder over accelerated storage. Food Chem. 2019, 295, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, D.K.; Kumar Singh, A.; Kumar, D.; Sharma, H. Effect of spray and freeze drying on physico-chemical, functional, moisture sorption and morphological characteristics of camel milk powder. LWT 2020, 134, 110117. [Google Scholar] [CrossRef]
- Zouari, A.; Briard-Bion, V.; Schuck, P.; Gaucheron, F.; Delaplace, G.; Attia, H.; Ayadi, M.A. Changes in physical and biochemical properties of spray dried camel and bovine milk powders. LWT 2020, 128, 109437. [Google Scholar] [CrossRef]
- Zouari, A.; Schuck, P.; Gaucheron, F.; Triki, M.; Delaplace, G.; Gauzelin-Gaiani, C.; Lopez, C.; Attia, H.; Ayadi, M.A. Microstructure and chemical composition of camel and cow milk powders’ surface. LWT 2020, 117, 108693. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Alhammadi, A.; Gharsallaoui, A.; Hamed, F.; Ghnimi, S. Physicochemical, rheological, and micro-structural properties of yogurts produced from mixtures of camel and bovine milks. NFS J. 2020, 19, 26–33. [Google Scholar] [CrossRef]
- Buchilina, A.; Aryana, K. Physicochemical and microbiological characteristics of camel milk yogurt as influenced by monk fruit sweetener. J. Dairy Sci. 2021, 104, 1484–1493. [Google Scholar] [CrossRef]
- Atwaa, E.H.; Hassan, M.A.A.; Ramadan, M.F. Production of probiotic stirred yoghurt from camel milk and oat milk. J. Food Dairy Sci. Mansoura Univ. 2020, 11, 259–264. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Zhang, N.; Zhang, W.; Ren, F. Improving the textural properties of camel milk acid gel by treatment with trisodium citrate and transglutaminase. LWT 2019, 103, 53–59. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Razavi, S.M.A. Influence of thermosonication treatment on the average size of fat globules, emulsion stability, rheological properties and color of camel milk cream. LWT 2020, 132, 109852. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Hakmani, H.; Al-Alawi, A.; Al-Marhub, I. Thermal characteristics of freeze-dried camel milk and its major components. Thermochim. Acta 2012, 549, 116–123. [Google Scholar] [CrossRef]
- Alhaj, O.A.; Taufik, E.; Handa, Y.; Fukuda, K.; Saito, T.; Urashima, T. Chemical characterization of oligosaccharides in commercially pasteurized dromedary camel (Camelus dromerarius) milk. Int. Dairy J. 2013, 28, 70–75. [Google Scholar] [CrossRef]
- Hassan, R.A.; El Zubeir, I.E.M.; Babiker, S.A. Effect of pasteurization of raw camel milk and storage temperature on the chemical composition of fermented camel milk. Int. J. Dairy Sci. 2007, 2, 166–171. [Google Scholar] [CrossRef] [Green Version]
- El Zubeir, I.E.M.; Marowa, I. Effect of pasteurization of milk on the keeping quality of fermented camel milk (Gariss) in Sudan. Livest. Res. Rural Dev. 2009, 21, 1–8. [Google Scholar]
- Rankin, S.A.; Christiansen, A.; Lee, W.; Banavara, D.S.; Lopez-Hernandez, A. Invited review: The application of alkaline phosphatase assays for the validation of milk product pasteurization. J. Dairy Sci. 2010, 93, 5538–5551. [Google Scholar] [CrossRef] [PubMed]
- Elagamy, E.I. Effect of heat treatment on camel milk proteins with respect to antimicrobial factors: A comparison with cows’ and buffalo milk proteins. Food Chem. 2000, 68, 227–232. [Google Scholar] [CrossRef]
- Loiseau, G.; Faye, B.; Serikbayeva, A.; Montet, D. Enzymes ability to serve as markers of pasteurized camel milk. In Proceedings of the Conference on New Horizons in Biotechnology, Trivandrum, India, 18–21 April 2001. [Google Scholar]
- Wernery, U.; Johnson, B.; George, R.M. Gamma-glutamyl transferase (GGT), a potential marker for the evaluation of heat treatment of dromedary milk. J. Camel Pract. Res. 2007, 14, 9. [Google Scholar]
- Lorenzen, P.C.; Wernery, R.; Johnson, B.; Jose, S.; Wernery, U. Evaluation of indigenous enzyme activities in raw and pasteurized camel milk. Small Rumin. Res. 2011, 97, 79–82. [Google Scholar] [CrossRef]
- Tayefi-Nasrabadi, H.; Hoseinpour-Fayzi, M.A.; Mohasseli, M. Effect of heat treatment on lactoperoxidase activity in camel milk: A comparison with bovine lactoperoxidase. Small Rumin. Res. 2011, 99, 187–190. [Google Scholar] [CrossRef]
- Kherouatou, N.; Nasri, M.; Attia, H. A study of the dromedary milk casein micelle and its changes during acidification. Braz. J. Food Technol. 2003, 6, 237–244. [Google Scholar]
- Lund, A.K.; Shah, A.H.; Jatoi, A.S.; Khaskheli, G.B.; Khaskheli, M.C.; Malhi, A.A.; Kalwar, M.A.; Khanzada, Q. Effect of heating on shelf life and sensory characteristics of camel milk. Pure Appl. Biol. 2020, 9, 74–79. [Google Scholar] [CrossRef]
- Felfoul, I.; Lopez, C.; Gaucheron, F.; Attia, H.; Ayadi, M.A. Fouling behavior of camel and cow milks under different heat treatments. Food Bioprocess Technol. 2015, 8, 1771–1778. [Google Scholar] [CrossRef]
- Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alzahrani, D.A.; Alrabiah, D.K.; AlYahya, S.A.; Alfadda, A.A. Proteomic profiling comparing the effects of different heat treatments on camel (Camelus dromedarius) milk whey proteins. Int. J. Mol. Sci. 2017, 18, 721. [Google Scholar] [CrossRef] [Green Version]
- Barthe, L. La composition du lait de chamelle. J. Pharm. Chim. 1905, 21, 386–388. [Google Scholar]
- Farah, Z. Effect of heat treatment on whey proteins of camel milk. Milchwissenschaft 1986, 41, 763–765. [Google Scholar]
- Farah, Z.; Atkins, D. Heat coagulation of camel milk. J. Dairy Res. 1992, 59, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Farah, Z.; Ruegg, M.W. The size distribution of casein micelles in camel milk. Food Microstruct. 1989, 52, 211–216. [Google Scholar]
- Attia, H.; Kherouatou, N.; Nasri, M.; Khorchani, T. Characterization of the dromedary milk casein micelle and study of its changes during acidification. Lait 2000, 80, 503–515. [Google Scholar] [CrossRef]
- Hattem, H.E.; Manal, A.N.; Hanna, S.S.; Elham, A.A. A study on the effect of thermal treatment on composition and some properties of camel milk. J. Brew. Distill. 2011, 2, 51–55. [Google Scholar]
- Saleh, S.K.; Mira, E.K.I.; Marzouk, N.M.; Abu-Zied, M.A. Effect of pasteurization on some chemical and bacteriological parameters on camel milk. Bull. Natl. Res. Cent. 2008, 33, 287–297. [Google Scholar]
- Beg, O.U.; von Bahr-Lindstrom, H.; Zaisi, Z.H.; Jornvall, H. Characterization of a heterogeneous camel milk whey non-casein protein. FEBS Lett. 1987, 216, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Momen, S.; Salami, M.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. The techno-functional properties of camel whey protein compared to bovine whey protein for fabrication a model high protein emulsion. LWT 2019, 101, 543–551. [Google Scholar] [CrossRef]
- Merin, U.; Bernstein, S.; Bloch-Damti, A.; Yagil, R.; van Creveld, C.; Lindner, P.; Gollop, N. A comparative study of milk serum proteins in camel (Camelus dromedaries) and bovine colostrums. Livest. Prod. Sci. 2001, 67, 297–301. [Google Scholar] [CrossRef]
- Ryskaliyeva, A.; Henry, C.; Faye, B.; Miranda, G.; Konuspayeva, G.; Martin, P. Combining different proteomic approaches to resolve complexity of the milk protein fraction of dromedary, Bactrian and hybrids from different regions of Kazakhstan. PLoS ONE 2018, 13, e0197026. [Google Scholar] [CrossRef] [Green Version]
- Genene, A.; Hansen, E.B.; Eshetu, M.; Hailu, Y.; Ipsen, R. Effect of heat treatment on denaturation of whey protein and resultant rennetability of camel milk. LWT 2019, 101, 404–409. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, J.; Zhao, D.; Liu, H.; Li, J.; Guo, M. Changes in chemical composition of Alxa Bactrian camel milk during lactation. J. Dairy Sci. 2005, 88, 3402–3410. [Google Scholar] [CrossRef]
- El-Hatmi, H.; Girardet, J.M.; Gaillard, J.L.; Yahyaoui, M.H.; Attia, H. Characterization of whey proteins of camel (Camelus dromedaries) milk and colostrums. Small Rumin. Res. 2007, 70, 267–271. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Loiseau, G.; Levieux, D. Lactoferrin and Immunoglobulin content in camel milk (C. bactrianus, C. dromedarius and hybrids) from Kazakhstan. J. Dairy Sci. 2007, 90, 38–46. [Google Scholar] [CrossRef]
- Levieux, D.; Levieux, A.; El-Hatmi, H.; Rigaudiere, J.P. Immunochemical quantification of heat denaturation of camel (Camelus dromedaries) whey protein. J. Dairy Res. 2006, 73, 421–430. [Google Scholar] [CrossRef]
- Konuspayeva, G. Camel milk composition and nutritional value. In Health and Environmental Benefits of Camel Products; AlHaj, O., Faye, B., Agrawal, R.D., Eds.; IGI Global: Delhi, PA, USA; Hershey: Delhi, PA, USA, 2020; pp. 363–378. [Google Scholar]
- Ryskaliyeva, A.; Henry, C.; Miranda, G.; Faye, B.; Konuspayeva, G.; Martin, P. The main WAP isoform usually found in camel milk arises from the usage of an improbable intron cryptic splice site in the precursor to mRNA in which a GC-AG intron occurs. BMC Genet. 2019, 20, 14. [Google Scholar] [CrossRef] [Green Version]
- Karray, N.; Lopez, C.; Lesieur, P.; Ollivon, M. Dromedary milk fat, thermal and structural properties 1. Crystalline forms obtained by slow cooling. Lait 2004, 80, 399–416. [Google Scholar] [CrossRef]
- Laleye, L.C.; Jobe, B.; Wasesa, A.A.H. Comparative study on heat stability and functionality of camel and bovine milk whey proteins. J. Dairy Sci. 2008, 91, 4527–4534. [Google Scholar] [CrossRef]
- Mehaia, M.A. Vitamin C and riboflavin content in camels’ milk, effects of heat treatments. Food Chem. 1994, 50, 153–155. [Google Scholar] [CrossRef]
- Alhaj, O.A.; Metwalli, A.M.; Elsayed, A. Heat stability of camel milk proteins after sterilisation process. J. Camel Pract. Res. 2011, 18, 277–282. [Google Scholar]
- Mehaia, M.A. Chemical composition of camel skim milk concentrated by ultrafiltration. Int. Dairy J. 1996, 6, 741–752. [Google Scholar] [CrossRef]
- Bukovics, S.; Hucker, A.; Szafner, G. Impact of microfiltration on camel milk’s microbiological and organoleptic characteristics. Sci. Pract. J. Vet. 2015, 2, 168–170. [Google Scholar]
- Sela, S.; Pinto, R.; Merin, U.; Rosen, B. Thermal inactivation of Escherichia coli in camel milk. J. Food Prot. 2003, 66, 1708–1711. [Google Scholar] [CrossRef]
- Al-Rasheedi, F.S.; Altulayan, B.S.; Al-Amre, S.S.; Faye, B.; Konuspayeva, G. Monitoring of camel milk quality in intensive dairy farm. In Proceedings of the 4th ISOCARD Conference, Almaty, Kazakhstan, 8–12 June 2015; Konuspayeva, G., Ed.; Special issue Journal Veterinariya, 2. Publ. ANTIGEN: Almaty, Kazakhstan, 2015; pp. 234–236. [Google Scholar]
- Abusheliabi, A.; Al-Rumaithi, H.O.; Olaimat, A.N.; Al-Nabuls, A.A.; Osaili, T.; Shaker, R.; Ayyash, M.M. Inhibitory effect of camel milk on Cronobacter sakazakii. J. Food Saf. 2016, 37. [Google Scholar] [CrossRef]
- Eissa, O.E.H.; Hamid, O.I.A. Quality characteristics of camel milk during different heat treatments. J. Agric. Vet. Sci. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; El-Zubeir, I.Y.M. Effect of heat treatment on keeping quality of camel milk. Ann. Food Sci. Technol. 2014, 15, 239–245. [Google Scholar]
- Fguiri, I.; Ziadi, M.; Abassi, M.; Arroum, S.; Khorchani, T. Suitability of camel milk to transformation in Leben by lactic starter. Afr. J. Microbiol. Res. 2012, 6, 7185–7192. [Google Scholar] [CrossRef]
- Khedid, K.; Faid, M.; Mokhtari, A.; Soulaumani, A.; Zinedine, A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol. Res. 2009, 164, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kadri, Z.; Spitaels, F.; Cnockaert, M.; Amar, M.; Joossens, M.; Vandamme, P. The bacterial diversity of raw Moroccan camel milk. Int. J. Food Microbiol. 2021, 341, 109050. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, M.; Al-Zoreky, N.S.; El-Dermerdash, H.A. Camel’s milk as a natural source for probiotics. Res. J. Microbiol. 2013, 8, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Abdou, A.M.; Hedia, R.H.; Omara, S.T.; Mahmoud, M.A.E.; Kandil, M.M.; Bakry, M.A. Interspecies comparison of probiotics isolated from different animals. Vet. World 2018, 11, 227–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yateem, A.; Balba, M.T.; Al-Surrayai, T.; Al-Mutairi, B.; Al-Daher, R. Isolation of lactic acid bacteria with probiotic potential from camel milk. Int. J. Dairy Sci. 2008, 3, 194–199. [Google Scholar] [CrossRef]
- Ider, S.; Belguesmia, Y.; Coucheney, F.; Kihal, M.; Drider, D. Impact of seasonality and environmental conditions on yeast diversity from camel’s milk collected in Algeria. Arch. Microbiol. 2019, 201, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Benmechernene, Z.; Chentouf, A.F.; Yahia, B.; Fatima, G.; Quintela-Baluja, M.; Calo-Mata, P.; Barros-Velázquez, J. Technological aptitude and applications of Leuconostoc mesenteroides bioactive strains isolated from Algerian raw camel milk. Biomed Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmeh, R.; Akbar, A.; Kishk, M.; Al-Onaizi, T.; Al-Shatti, A.; Shajan, A.; Akbar, B.; Al-Mutairi, S.; Yateem, A. Characterization of semipurified enterocin produced by Enterococcus faecium strains isolated from raw camel milk. J. Dairy Sci. 2018, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abo-Amer, A.R. Inhibition of foodborn pathogens by a bacteriocin-like substance produced by a novel strain of Lactobaccilus acidophilus isolated from camel milk. Appl. Biochem. Microbiol. 2013, 49, 270–279. [Google Scholar] [CrossRef]
- Davati, N.; Yazdi, F.T.; Zibaee, S.; Shahidi, F.; Reza Edalatian, M. Study of lactic acid bacteria community from raw milk of Iranian one humped camel and evaluation of their probiotic properties. Jundishapur J. Micriobiol. 2014, 8, e16750. [Google Scholar] [CrossRef] [Green Version]
- Jans, C.; Meile, L.; Kaindi, D.W.M.; Kogi-Makau, W.; Lamuka, P.; Renault, P.; Kreikemeyer, B.; Lacroix, C.; Hattendorf, J.; Zinsstag, J.; et al. African fermented dairy products—Overview of predominant technologically important microorganisms focusing on African Streptococcus infantarius variants and potential future applications for enhanced food safety and security. Int. J. Food Microbiol. 2017, 250, 27–36. [Google Scholar] [CrossRef]
- Kaindi, D.W.M.; Njage, P.K. Microbial aspect of lactic acid bacteria isolated from camel milk. In Health and Environmental Benefits of Camel Products; Alhaj, O.A., Faye, B., Agrawal, R.P., Eds.; IGI Global: Delhi, PA, USA; Hershey: Delhi, PA, USA, 2020; pp. 54–74. [Google Scholar]
- Baubekova, A.; Akhmetsadykova, S.; Konuspayeva, G.; Akhmetsadykov, N.; Faye, B.; Loiseau, G. Biodiversity study of the yeasts in fresh and fermented camel and mare milk by denaturating gradient gel electrophoresis. J. Camel Pract. Res. 2015, 22, 91–95. [Google Scholar] [CrossRef]
- Akhmetsadykova, S.; Baubekova, A.; Konuspayeva, G.; Akhmetsadykov, N.; Faye, B.; Loiseau, G. Lactic acid bacteria biodiversity in raw and fermented camel milk. Afr. J. Food Sci. Technol. 2015, 6, 84–88. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, H.; Kwok, L.Y.; Guo, F.; Ji, R.; Ya, M.; Chen, Y. Analyses of phys.;icochemical properties, bacterial microbiota, and lactic acid bacteria of fresh camel milk collected in Inner Mongolia. J. Dairy Sci. 2019, 103, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Agamy, E.I.; Ruppanner, R.; Ismail, A.; Champagne, C.P.; Assaf, R. Antibacterial and antiviral activity of camel milk protective proteins. J. Dairy Res. 1992, 59, 169–175. [Google Scholar] [CrossRef]
- Berhe, T.; Ipsen, R.; Seifu, E.; Kurtu, M.Y.; Eshetu, M.; Hansen, E.B. Comparison of the acidification activities of commercial starter cultures in camel and bovine milk. LWT 2017, 89, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Benyagoub, E.; Ayat, M. Biochemical, physico-chemical and microbiological properties of camel raw milk marketed in Bechar city (South-West Algeria), hygienic and safe consumers’ approach. Microbes Health 2015, 4, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Elhaj, A.E.; Somaya, F.A.B.; Mohamed, T.T. Aerobic bacteria and fungi associated with raw camel’s milk. Online J. Anim. Feed Res. 2014, 4, 15–17. [Google Scholar]
- Jrad, Z.; El-Hatmi, H.; Fguiri, I.; Arroum, S.; Assadi, M.; Khorchani, T. Antibacterial activity of lactic acid bacteria isolated from Tunisian camel milk. Afr. J. Microbiol. Res. 2013, 7, 1002–1008. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B. Identité, vertus thérapeutiques et al.légation santé, les produits fermentés d’Asie Centrale. In Culture des laits du Monde; Les Cahiers de l’OCHA n°15: Paris, France, 2011; pp. 135–145. [Google Scholar]
- Rahman, N.; Xiaohong, C.; Meiqin, F.; Mingsheng, D. Characterization of the dominant microflora in naturally fermented camel milk shubat. World J. Microbiol. Biotechnol. 2009, 25, 1941–1946. [Google Scholar] [CrossRef]
- Guo, L.; Xu, W.; Li, C.; Guo, Y.; Chagan, I. Comparative study of physicochemical composition and microbial community of Khoormog, Chigee, and Airag, traditionally fermented dairy products from Xilin Gol in China. Food Sci. Nutr. 2021, 9, 1564–1573. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Mohamed, B.E.; Yousif, N.M.E.; Faye, B.; Loiseau, G. Antimicrobial activity and antibiotic resistance of LAB isolated from Sudanese traditional fermented camel (Camelus dromedarius) milk gariss. Int. J. Biosci. 2012, 2, 129–136. [Google Scholar]
- Adelgadir, W.S.; Ahmed, T.K.; Dirar, H.A. The traditional fermented milk products of the Sudan. Int. J. Food Microbiol. 1998, 44, 1–13. [Google Scholar] [CrossRef]
- Maitha, M.; Kaindi, D.W.M.; Wangoh, J.; Mbugua, S. Microbial quality and safety of traditional fermented camel milk product suusac sampled from different regions in North Eastern, Kenya. Asian Food Sci. J. 2019, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Algruin, K.; Konuspayeva, G. Making Leben with Camel Milk. In Proceedings of the 4th ISOCARD Conference, Almaty, Kazakhstan, 8–12 June 2015; Konuspayeva, G., Ed.; Special issue Journal Veterinariya, 2. Antigen: Almaty, Kazakhstan, 2015; p. 393. [Google Scholar]
- Seifu, E.; Abraham, A.; Kurtu, M.Y.; Yilma, Z. Isolation and characterization of lactic acid bacteria from Ititu, Ethiopian traditional fermented camel milk. J. Camelid Sci. 2012, 5, 82–98. [Google Scholar]
- Berhe, T.; Ipsen, R.; Seifu, E.; Kurtu, M.Y.; Fugl, A.; Hansen, E.B. Metagenomic analysis of bacterial community composition in Dhanaan, Ethiopian traditional fermented camel milk. FEMS Microbiol. Lett. 2019, 366, fnz128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulay, S. Alimentation, Diététique et Relations Sociales au Sahara, L’exemple des Pasteurs Nomades Maures de Mauritanie. 2008. Available online: www.lemangeur-ocha.com (accessed on 23 February 2021).
- Ismaili, M.A.; Hamama, A.; Saidi, B.; Zahar, M.; Meryem, A. Chemical composition, microbial profile and identification of lactic bacteria of Moroccan fermented camel milk Lfrik. Curr. Res. Nutr. Food Sci. 2017, 5, 383–390. [Google Scholar] [CrossRef]
- Soleymanzadeh, N.; Mirdamadi, S.; Kianirad, M. Antioxidant activity of camel and bovine milk fermented by lactic acid bacteria isolated from traditional fermented camel milk (Chal). Dairy Sci. Technol. 2016, 96, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Lore, T.A.; Mbugua, S.K.; Wangoh, J. Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product. LWT 2005, 38, 125–130. [Google Scholar] [CrossRef]
- Jans, C.; Bugnard, J.; Njage, P.M.K.; Lacroix, C.; Miele, L. Lactic acid bacteria diversity of African raw and fermented camel milk products reveals a highly competitive, potentially health-threatening predominant microflora. LWT Food Sci. Technol. 2012, 47, 371–379. [Google Scholar] [CrossRef]
- Adelgadir, W.; Nielsen, D.S.; Hamad, S.; Jakobsen, M. A traditional Sudanese fermented camel’s milk product, Gariss, as a habitat of Streptococcus infantarius subsp. infantarius. Int. J. Food Microbiol. 2008, 127, 215–219. [Google Scholar] [CrossRef]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Akhmetsadykova, S.; Baubekova, A.; Konuspayeva, G.; Akhmetsadykov, N.; Loiseau, G. Microflora identification of fresh and fermented camel milk from Kazakhstan. Emir. J. Food Agric. 2014, 26, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Ashmaig, A.; Hasan, A.; El Gaali, E. Identification of lactic acid bacteria isolated from traditional Sudanese fermented camel milk (Garris). Afr. J. Microbiol. Res. 2009, 3, 451–457. [Google Scholar]
- Berzhanova, R.; Sartaeva, A.; Sagyndykov, U.; Mukasheva, T.; Shigaeva, M. The studying of diversity of lactic microorganisms isolated from shubat of various areas of Kazakhstan. J. Biotechnol. 2014, 185, S82. [Google Scholar] [CrossRef]
- Njage, P. Microbial Diversity, Safety and Role of Predominant Lactic Acid Bacteria in Raw and Spontaneously Fermented Camel Milk from Kenya and Somalia. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2010. [Google Scholar]
- Baubekova, A.; Konuspayeva, G.; Akhmetsadykova, S.H.; Akhmetsadykov, N. Пoдгoтoвка прoмышленнoгo прoизвoдства заквасoк– выделение и идентификация бактерий для кумыса и шубата. [Preparation of industrial production of starter cultures—isolation and identification of bacteria from kumis and shubat]. Вестник КазНУ Серия биoлoгическая 2014, 60, 178–181. [Google Scholar]
- Faye, B.; Konuspayeva, G. Innovations in camel milk processing, The new challenges for marketing camel dairy products and the consequences on genetic selection. In Proceedings of the Regional Conference for Animal Genetic Resources Conservation, towards Sustainable Utilization, Muscat, Oman, 23–24 February 2016; Available online: https://oapgrc.gov.om/Documents/The%20new%20challenges%20for%20marketing%20camel%20dairy%20products%20and%20the%20consequences%20on%20genetic%20selection_Bernard%20Fye.pdf (accessed on 23 February 2021).
- Abdel Rahman, I.E.; Dirar, H.A.; Osman, M.A. Microbiological and biochemical changes and sensory evaluation of camel milk fermented by selected bacterial starter cultures. Afr. J. Food Sci. 2009, 3, 398–4059. [Google Scholar]
- Ayyash, M.; Al-Dhaheri, A.S.; Al-Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic, A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef] [Green Version]
- Dugan, F.M. Fermentive microorganisms in the Prehistory of Europe, the steppes, and Indo-Iranian Asia and their contemporary use in traditional and probiotic beverages. Fungi 2009, 2, 16–39. [Google Scholar]
- Kappeler, S.R.; van den Brink, H.M.; Rahbek-Nielsen, H.; Farah, Z.; Puhan, Z.; Hansen, E.B.; Johansen, E. Characterizations of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk. Biochem. Biophys. Res. Commun. 2006, 342, 647–654. [Google Scholar] [CrossRef]
- Konuspayeva, G. Manufacture and Challenges of Camel Milk Cheese. In Health and Environmental Benefits of Camel Products; Alhaj, O.A., Faye, B., Agrawal, R.P., Eds.; IGI Global: Delhi, PA, USA; Hershey: Delhi, PA, USA, 2020; pp. 110–122. [Google Scholar]
- Ramet, J.P. L’aptitude fromagère du lait de dromadaire. Rev. D’elevage Et Médecine Vétérinaire Des Pays Trop. 1989, 42, 105–111. [Google Scholar] [CrossRef]
- Hailu, Y.; Seifu, E.; Yilma, Z. Physicochemical properties and consumer acceptability of soft unripened cheese made from camel milk using crude extract of ginger (Zingiber officinale) as coagulant. Afr. J. Food Sci. 2014, 8, 87–91. [Google Scholar]
- Terefe, M.A.; Kebede, A.; Kebede, M. Clotting Activities of Partially Purified Extracts of Moringa oleifera L. on Dromedary Camel Milk. East Afr. J. Sci. 2017, 11, 117–128. [Google Scholar]
- Boudjenah-Haroun, S.; Laleye, L.; Moulti-Mati, F.; Si Ahmed, S.; Mahboub, N.; Siboukeur, O.E.; Mati, A. Comparative study of milk clotting activity of crude gastric enzymes extracted from camels’ abomasum at different ages and commercial enzymes (rennet and pepsin) on bovine and camel milk. Emir. J. Food Agric. 2011, 23, 301–310. [Google Scholar]
- Jones-Abeiderrhamane, N. Camel Cheese, Seemed like a Good Idea; Jones-Abeiderrahmane, N., Ed.; JA Publ.: Nouakchott, Mauritania, 2013; 387p. [Google Scholar]
- Konuspayeva, G.; Faye, B.; Baubekova, A.; Loiseau, G. Camel gruyere cheese making. In Proceedings of the 3rd ISOCARD Conference, Muscat, Oman, 29 January–1 February 2012; Johnson, E.H., Maghoub, O., Eljack, A., Kadim, I., Bobabe, P.A., Tageldin, M.H., Almarzooqi, W.S., Eltahir Ahmed, Y., Eds.; Sultan Qaboos University: Muscat, Oman, 2012; pp. 218–219. [Google Scholar]
- Konuspayeva, G.; Camier, B.; Gaucheron, F.; Faye, B. Some parameters to process camel milk into cheese. Emir. J. Food Agric. 2014, 26, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Konuspayeva, G.; Camier, B.; Aleilawi, N.; Al-Shumeimyri, M.; Algruin, K.; Alshammari, F.; Beaucher, E.; Faye, B. Manufacture of dry- and brine-salted soft camel cheeses for the camel dairy industry. Int. J. Dairy Technol. 2017, 70, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Shahein, M.R.; Hassanein, A.M.; Zayan, A.F. Evaluation of soft cheese manufactured from camel and buffalo milk. World J. Dairy Food Sci. 2014, 9, 213–219. [Google Scholar]
- Hailu, Y.; Hansen, E.B.; Eshetu, M.; Ipsen, R. Factors influencing the gelation and rennetability of camel milk using camel chymosin. Int. Dairy J. 2016, 60, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Soltani, M.; Boran, O.S.; Hayaloglu, A.A. Effect of various blends of camel chymosin and microbial rennet (Rhizomucor miehei) on microstructure and rheological properties of Iranian UF White cheese. LWT Food Sci. Technol. 2016, 68, 724–728. [Google Scholar] [CrossRef]
- Mohammed, S.; Eshetu, M.; Tadesse, Y.; Hailu, Y. Rheological properties and shelf life of soft cheese made from camel milk using camel chymosin. J. Dairy Vet. Sci. 2019, 10, 555794. [Google Scholar] [CrossRef]
- Kamal, M.; Foukani, M.; Karoui, R. Effects of heating and calcium and phosphate mineral supplementation on the physical properties of rennet-induced coagulation of camel and cow milk gels. J. Dairy Res. 2017, 84, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Farah, Z.; Bachmann, M.R. Rennet coagulation properties of camel milk. Milchwissenschaft 1987, 42, 689–692. [Google Scholar]
- Wolfschoon, A. Influence of calcium chloride addition to milk on the cheese yield. Int. Dairy J. 1997, 7, 249–254. [Google Scholar] [CrossRef]
- Gioacchini, A.M.; De Santi, M.; Guescini, M.; Brandi, G.; Stocchi, V. Characterization of the volatile organic compounds of Italian ‘Fossa’ cheese by solid-phase microextraction gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, S.M.S.; Khalifa, S.A. Manufacture of processed cheese spread from camel cheese based, Evaluation of cheese characteristics. Am. J. Food Sci. Nutr. Res. 2018, 5, 76–86. [Google Scholar]
- El-Zubeir, I.E.M.; Jabreel, S.O. Fresh cheese from camel milk coagulated with Camifloc. Int. J. Dairy Technol. 2008, 61, 90–95. [Google Scholar] [CrossRef]
- El-Gendy, M.H. Impact of manufacturing processes on the industry soft cheese from camel milk. J. Biol. Chem. Environ. Sci. 2018, 13, 491–510. [Google Scholar]
- Faye, B.; Jaouad, J.; Bhrawi, K.; Senoussi, A.; Bengoumi, M. Elevage camelin en Afrique du Nord, état des lieux et perspectives. Rev. D’elevage Méd. Vétérinaire Pays. Trop. 2014, 67, 213–221. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Khalifa, S.A. Effect of freeze drying on camel’s milk nutritional properties. Int. Food Res. J. 2015, 22, 1438–1445. [Google Scholar]
- Sulieman, A.M.E.; Elamin, O.; Elkhalifa, E.A.; Laleye, L. Comparison of Physicochemical Properties of Spray-dried Camel’s Milk and Cow’s Milk Powder. Int. J. Food Sci. Nutr. Eng. 2014, 4, 15–19. [Google Scholar] [CrossRef]
- Zouari, A.; Marchesseau, S.; Chevalier-Lucia, D.; Raffard, G.; Ayadi, M.A.; Picart-Palmade, L. Acid gelation of raw and reconstituted spray-dried dromedary milk, a dynamic approach of gel structuring. Int. Dairy J. 2018, 81, 95–103. [Google Scholar] [CrossRef]
- Habtegebriel, H.; Wawire, M.; Sila, D. The Effect of Pretreatment (Spray Drying) on the Yield and Selected Nutritional Components of Whole Camel Milk Powder. J. Food Sci. 2018, 83, 2983–2991. [Google Scholar] [CrossRef]
- Vega, C.; Roos, Y.H. Invited review, Spray-dried dairy and dairy-like emulsions-Compositional considerations. J. Dairy Sci. 2006, 89, 383–401. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, S.A.; Ibrahim, A.H. Influence of addition modified starches as stabilizer on physicochemical and textural properties of camel’s milk yoghurt. Zagazig J. Agric. Res. 2015, 42, 13–26. [Google Scholar]
- Abd-Elhamid, A.M.; Elbayoumi, M.M. Effect of heat treatment and fermentation on bioactive behavior in yoghurt made from camel milk. Am. J. Food Sci. Technol. 2017, 5, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Afolabi, L.; Onilude, H.; Afolabi, M.O. Assessment of microbiological quality of yogurt sold by street vendors in Onitsha metropolis, Anambra state, Nigeria. Br. Microbiol. Res. J. 2013, 3, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Gorelik, O.; Shatskikh, Y.; Rebezov, M.; Kanareikin, V.; Lihodeyevskaya, O.; Okuskhanova, E. Study of Chemical and Mineral Composition of New Sour Milk Bio-product with Sapropel Powder. Annu. Res. Rev. Biol. 2017, 18, 1–5. [Google Scholar] [CrossRef]
- Berhe, T.; Seifu, E.; Ipsen, R.; Kurtu, M.Y.; Hansen, E.B. Processing challenges and opportunities of camel dairy products. Int. J. Food Sci. 2017, 2, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumah, R.Y.; Shaker, R.R.; Abu-Jdayil, B. Effect of milk source on the rheological properties of yogurt during the gelation process. Int. J. Dairy Technol. 2001, 54, 89–93. [Google Scholar] [CrossRef]
- Attia, H.; Dhouib, N. Dromedary milk lactic acid fermentation, Microbiological and rheological characteristics. Microb. Biotechnol. 2001, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lajnaf, R.; Picart-Palmade, L.; Attia, H.; Marchesseau, S.; Ayadi, M.A. Foaming and adsorption behavior of bovine and camel proteins mixed layers at the air/water interface. Colloids Surf. B Biointerfaces 2016, 151, 287–294. [Google Scholar] [CrossRef]
- Hashim, I.B.; Khalil, A.H.; Habib, H. Quality and acceptability of a set-type yogurt made from camel milk. J. Dairy Sci. 2009, 92, 857–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shegaw, A.; Ipsen, R.; Kurtu, M.Y.; Eshetu, M.; Hansen, E.B.; Hailu, Y.; Waktola, A.; Dashe, D. Development of yogurt from camel milk using Exopolysaccharide producing lactic acid bacteria. Ethiop. J. Anim. Prod. 2020, 20, 29–43. [Google Scholar]
- Elsahoryi, N.A.; Al-Sayyed, H.F. Manufacture of dairy and non-dairy camel milk products. Chap. 5. In Health and Environmental Benefits of Camel Products; Alhaj, O.A., Faye, B., Agrawal, R.P., Eds.; IGI Global: Delhi, PA, USA; Hershey: Delhi, PA, USA, 2020; pp. 75–109. [Google Scholar]
- Shamsia, S.M. Nutritional and therapeutic properties of camel and human milks. Int. J. Genet. Mol. Biol. 2009, 1, 52–58. [Google Scholar]
- Mohsin, A.; Ni, H.; Luo, Y.; Wei, Y.; Tian, X.; Guan, W.; Ali, M.; Mahmood Khan, I.; Niazi, S.; Rehman, S.; et al. Qualitative improvement of camel milk date yoghurt by addition of biosynthesized xanthan from orange waste. LWT 2019, 108, 61–68. [Google Scholar] [CrossRef]
- Galeboe, O.; Seifu, E.; Sekwati-Monang, B. Production of camel milk yoghurt, Physicochemical and microbiological quality and consumer acceptability. Int. J. Food Stud. 2018, 7, 51–63. [Google Scholar] [CrossRef]
- Kavas, N.; Kavas, G. Probiotic frozen yoghurt production using camel milk (Camelus dromedarius) with improved functions by strawberry guava (Psidium littorale var. cattleianum) fortification. Br. J. Appl. Sci. Technol. 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Ahmed, S.; Haroun, R.; Eisa, M. Banana frozen yogurt from camel milk. Pak. J. Nutr. 2010, 9, 955. [Google Scholar] [CrossRef] [Green Version]
- Konuspayeva, G.; Lemarie, E.; Faye, B.; Loiseau, G.; Montet, D. Fatty acid and cholesterol composition of camel’s (Camelus bactrianus, Camelus dromedarius and hybrids) milk in Kazakhstan. Dairy Sci. Technol. 2008, 88, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Attia, H.; Kherouatou, N.; Fakhfakh, N.; Khorchani, T.; Trigui, N. Dromedary milk fat, biochemical, microscopic and rheological characteristics. J. Food Lipids 2000, 7, 95–112. [Google Scholar] [CrossRef]
- Berhe, T.; Seifu, E.; Kurtu, M.Y. Physicochemical properties of butter made from camel milk. Int. Dairy J. 2013, 31, 51–54. [Google Scholar] [CrossRef]
- Farah, Z.; Streiff, T.; Bachmann, M.R. Manufacture and characterization of camel milk butter. Milchwissenschaft 1989, 44, 412–414. [Google Scholar]
- Parmar, N.B. Characterization of Ghee Prepared from Camel Milk and Evaluation of Its Shelf Life during Storage. Master’s Thesis, SMC College of Dairy Science, Anand Agricultural University, Anand, Gujarat, India, 2013. [Google Scholar]
- Ahmed, A.S.M.; El-Zubeir, I.E.M. Microbiological and sensory properties of low-fat ice cream from camel milk using natural additives. Ann. Food Sci. Technol. 2015, 16, 236–244. [Google Scholar]
- Hashim, I. Acceptance of camel milk among elementary school students in Al-Ain City, United Arab Emirates. Emir. J. Food Agric. 2017, 17, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Maryniak, N.; Hansen, E.; Ballegaard, A.S.; Sancho, A.; Bogh, K. Comparison of the Allergenicity and Immunogenicity of Camel and Cow’s Milk—A Study in Brown Norway Rats. Nutrients 2018, 10, 1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Processing | Reference | Country | Topic |
|---|---|---|---|
| Pasteurization | Zhang et al., 2020 [7] | China | Fouling characterization |
| Lajnaf et al., 2020 [8] | Tunisia | Foaming properties | |
| Li et al., 2020 [9] | China | Protein profile | |
| Yehia et al., 2020 [10] | KSA | Heat-resistant Staphylococcus aureus | |
| Bragason et al., 2020 [11] | Ethiopia | Antibacterial properties | |
| Fermentation | Gammoh et al., 2020 [12] | Jordan | Modification of functional properties |
| Sobti et al., 2021 [13] | UAE | Effect of pectin and alginate | |
| Mortazavi et al., 2021 [14] | Iran | Enrichment of fermented milk with pomegranate peel | |
| Zhadyra et al., 2021 [15] | China | Microflora diversity | |
| Sharma et al., 2021 [16] | KSA | Probiotic properties | |
| Ayyash et al., 2021 [17] | UAE | Exopolysaccharide impact | |
| Soleymanzadeh, 2019 [18] | Iran | Novel β-casein in fermented milk | |
| Edalati et al., 2019 [19] | Iran | Antagonistic effects of probiotic bacteria | |
| Cheese | Mbye et al., 2020 [20] | UAE | Physicochemical properties, sensory quality, and coagulation behavior |
| Belkheir et al., 2020 [21] | Algeria | Effect of starter on volatile and sensory profiles | |
| El-Hatmi et al., 2020 [22] | Tunisia | Cheese fortification by Allium roseum | |
| Spray-dried and freeze-dried | Perusko et al., 2021 [23] | Serbia | Maillard reaction products |
| Ho et al., 2019 [24] | Australia | Physicochemical properties with accelerated storage | |
| Deshval et al., 2020 [25] | India | Functional and morphological properties | |
| Zouari et al., 2020 [26] | Tunisia | Physical and biochemical properties | |
| Zouari et al., 2020 [27] | Tunisia | Microstructure and chemical composition | |
| Yoghurt | Kamal-Eldin et al., 2020 [28] | UAE | Properties of mixed camel/cow yoghurt |
| Buchilina and Aryana, 2021 [29] | USA | Yoghurt with monk fruit | |
| Atwaa et al., 2020 [30] | Egypt | Production of stirred camel yogurt | |
| Chen et al., 2019 [31] | China | Trisodium citrate and transglutaminase treatment of acid gel | |
| Cream | Kashaninejad and Razavi, 2020 [32] | Iran | Properties and color |
| Product | References | TVM | LAB | YM | Coli. | pH |
|---|---|---|---|---|---|---|
| In log10 CFU/mL | ||||||
| Suusac | Lore et al. [109] | 9.03 | 6.77 | 2.05 | 1.00 | 6.0–4.25 |
| Jans et al. [110] | ND | 7.2–8.5 | ND | ND | 4.9 | |
| Garris | Hassan et al. [35] | ND | ND | ND | ND | 6.2–3.8 |
| Adelgadir et al. [111] | 7.11–8.36 | 7.34–8.66 | 6.05–7.79 | ND | 3.79–4.32 | |
| Shubat | Rahman et al. [98] | ND | 6.8–7.6 | 4.3–4.7 | ND | ND |
| Process | Activity | Particularities | Comments |
|---|---|---|---|
| Raw camel milk (raw material) | Limit the number of suppliers or implement collecting centers with quality control | Low bacterial load (ideally <100 CFU/mL coliforms) Titratable acidity <16° Dornic | Without respect for hygiene, the milk may clot during powder processing and cause harmful fouling of the equipment |
| Concentration to remove some of the water (on average, there is 88% water in camel milk) | Remove at least 30% of the water using the principle of a “pressure cooker”, which is less expensive in terms of energy, saving atomization time and improving the quality of the final product | If hygienic standards are insufficient, risk of clotting | Clotted products are difficult to reuse, not only for the reconstruction of liquid milk, but also for the transformation into other products (cheese, yoghurt, fermented milk) |
| Homogenization to better emulsify | Make smaller fat and casein micelles to obtain a good-quality emulsion | Optional for better powder quality, a stabilizer can be added to the emulsion (caseinates), and lactose can possibly be removed to obtain an optimal 1:1 ratio of caseinates/lactose | This noncompulsory phase would result in a powder of better physical quality (solubility, fluidity, and low hygroscopic capacity) and stability over a longer period (preservation time) |
| Spray-drying | Optimize temperature (inlet/outlet), atomization pressure, and input flow to minimize energy costs and preserve the nutritional properties of camel milk | If hygienic standards are insufficient, the risk of appearance of poorly dried agglomerates will impact the commercial quality of the product | Data are available from suppliers |
| Packaging of camel milk powder | To preserve the quality of the finished product, it is advisable to use automated bagging | Working in a sterile atmosphere under strictly controlled humidity | This is an important step because any anomaly can involve all the previous steps (critical point in a Hazard Analysis Critical Control Point (HACCP) approach) Access to this part of the chain should be limited |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konuspayeva, G.; Faye, B. Recent Advances in Camel Milk Processing. Animals 2021, 11, 1045. https://doi.org/10.3390/ani11041045
Konuspayeva G, Faye B. Recent Advances in Camel Milk Processing. Animals. 2021; 11(4):1045. https://doi.org/10.3390/ani11041045
Chicago/Turabian StyleKonuspayeva, Gaukhar, and Bernard Faye. 2021. "Recent Advances in Camel Milk Processing" Animals 11, no. 4: 1045. https://doi.org/10.3390/ani11041045
APA StyleKonuspayeva, G., & Faye, B. (2021). Recent Advances in Camel Milk Processing. Animals, 11(4), 1045. https://doi.org/10.3390/ani11041045