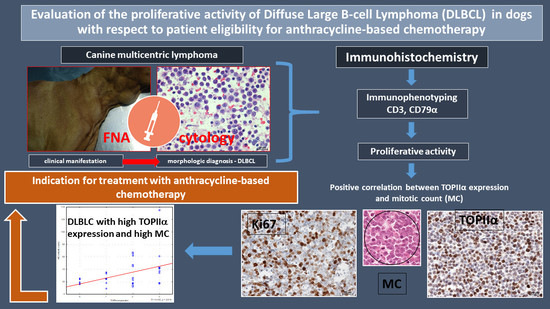
Evaluation of the Proliferative Activity of Diffuse Large B-Cell Lymphoma (DLBCL) in Dogs with Respect to Patient Eligibility for Anthracycline-Based Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Material for the Study
2.2. Histology and Immunohistochemistry
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grüntzig, K.; Graf, R.; Boo, G.; Guscetti, F.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Folkers, G.; et al. Swiss Canine Cancer Registry 1955–2008. Occurrence of the most common tumour diagnoses and influence of age, breed, body size, sex and neutering status on tumour development. J. Comp. Pathol. 2016, 155, 56–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vail, D.; Pinkerton, M.; Young, K. Withrow & MacEwen’s Small Animal Clinical Oncology Hematopoietic Tumours, 5th ed.; Elsevier: St Louis, MO, USA, 2013; p. 608. [Google Scholar]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer incidence in pet dogs: Findings of the animal tumor registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef]
- Aresu, L.; Martini, V.; Rossi, F.; Vignoli, M.; Sampaolo, M.; Aricò, A.; Laganga, P.; Pierini, A.; Frayssinet, P.; Mantovani, R.; et al. Canine indolent and aggressive lymphoma: Clinical spectrum with histologic correlation. Vet. Comp. Oncol. 2015, 13, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M. Canine lymphoma: A review. Vet. Quart. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- Valli, V.E.; San Myint, M.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of canine malignant lymphomas according to the world health organization criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Curran, K.M.; Schaffer, P.A.; Frank, C.B.; Lana, S.E.; Hamil, L.E.; Burton, J.H.; Labadie, J.; Ehrhart, E.J.; Avery, P.R. BCL2 and MYC are expressed at high levels in canine diffuse large B-cell lymphoma but are not predictive for outcome in dogs treated with CHOP chemotherapy. Vet. Comp. Oncol. 2017, 15, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Rebhun, R.B.; Lana, S.E.; Ehrhart, E.J.; Charles, J.B.; Thamm, D.H. Comparative analysis of survivin expression in untreated and relapsed canine lymphoma. J. Vet. Intern. Med. 2008, 22, 989–995. [Google Scholar] [CrossRef]
- Childress, M.O.; Ramos-Vara, J.A.; Ruple, A. Retrospective analysis of factors affecting clinical outcome following CHOP-based chemotherapy in dogs with primary nodal diffuse large B-cell lymphoma. Vet. Comp. Oncol. 2018, 16, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ponce, F.; Magnol, J.; Ledieu, D.; Marchal, T.; Turinelli, V.; Chalvet-Monfray, K.; Fournel-Fleury, C. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet. J. 2004, 167, 158–166. [Google Scholar] [CrossRef]
- Valli, V.E.; Kass, P.H.; San Myint, M.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef]
- Sapierzyński, R.; Kliczkowska-Klarowicz, K.; Jankowska, U.; Jagielski, D. Cytodiagnostics of canine lymphomas—Possibilities and limitations. Pol. J. Vet. Sci. 2016, 19, 433–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienzle, D.; Vernau, W. The diagnostic assessment of canine lymphoma: Implications for treatment. Clin. Lab. Med. 2011, 31, 21–39. [Google Scholar] [CrossRef]
- Sayag, D.; Fournel-Fleury, C.; Ponce, F. Prognostic significance of morphotypes in canine lymphomas: A systematic review of literaturę. Vet. Comp. Oncol. 2018, 16, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, S.M.; Keller, B.; Grest, P.; Börger, C.T.; Guscetti, F. Validation of tissue microarrays for immunohistochemical analyses of canine lymphomas. J. Vet. Diagn. Investig. 2007, 19, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiupel, M.; Teske, E.; Bostock, D. Prognostic factors for treated canine malignant lymphoma. Vet. Pathol. 1999, 36, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Sierra, M.O.R.; Santilli, J.; Anai, L.A.; Da Silva, M.C.L.; Sueiro, F.A. Prognostic Significance of Ki67 and Its Correlation with Mitotic Index in Dogs with Diffuse Large B-Cell Lymphoma Treated with 19-Week CHOP-Based Protocol. J. Vet. Diag. Investig. 2018, 30, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Legendre, A.M. Treatment of Dogs with Lymphoma: A Work in Progress. J. Vet. Intern. Med. 2007, 21, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.; Thamm, D.H. Retrospective analysis for treatment of naïve canine multicentric lymphoma with a 15-week, maintenance-free CHOP protocol. Vet. Comp. Oncol. 2016, 1, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Flory, A.B.; Rassnick, K.M.; Erb, H.N.; Garrett, L.D.; Northrup, N.C.; Selting, K.A. Evaluation of factors associated with second remission in dogs with lymphoma undergoing retreatment with a cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy protocol: 95 cases (2000–2007). J. Am. Vet. Med. Assoc. 2011, 238, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Daters, A.T.; Mauldin, G.E.; Mauldin, G.N.; Brodsky, E.M.; Post, G.S. Evaluation of a multidrug chemotherapy protocol with mitoxantrone based maintenance (CHOP-MA) for the treatment of canine lymphoma. Vet. Comp. Oncol. 2009, 8, 11–22. [Google Scholar] [CrossRef]
- Rassnick, K.; Bailey, D.; Malone, E. Comparison between L-CHOP and an L-CHOP protocol with interposed treatments of CCNU and MOPP (L-CHOP-CCNU-MOPP) for lymphoma in dogs. Vet. Comp. Oncol. 2010, 8, 243–253. [Google Scholar] [CrossRef]
- Simon, D.; Nolte, I.; Eberle, N. Treatment of dogs with lymphoma using a 12-week, maintenance-free combination chemotherapy protocol. J. Vet. Intern. Med. 2006, 20, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.; Garrett, L.; Vail, D. Evaluation of a high-dose chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J. Vet. Intern. Med. 2000, 14, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.R.; Couto, C.G.; Wittum, T.E. Factors influencing first remission and survival in 145 dogs with lymphoma: A retrospective study. J. Am. Anim. Hosp. Assoc. 2000, 36, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Hajduk, M.; Olszewski, W.P.; Smietana, A. Evaluation of the predictive value of topoisomerase II alpha in patients with breast carcinoma. Pol. J. Pathol. 2009, 60, 115–123. [Google Scholar]
- Sosinska-Mielcarek, K.; Jassem, J. Predictive role of topoisomerase IIα expression in anthracycline based breast cancer chemotherapy. J. Oncol. 2005, 55, 252–256. [Google Scholar]
- Villman, K.; Sjöström, J.; Heikkilä, R.; Hultborn, R.; Malmström, P.; Bengtsson, N.O. TOP2A and HER2 gene amplification as predictors of response to anthracycline treatment in breast cancer. Acta Oncol. 2006, 45, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Al-Nadaf, S.; Rebhun, R.B.; Curran, K.M. Retrospective analysis of doxorubicin and prednisone as first-line therapy for canine B-cell lymphoma. BMC Vet. Res. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Beaver, L.; Strottner, G.; Klein, M. Response rate after administration of a single dose of doxorubicin in dogs with B-cell or T-cell lymphoma: 41 cases (2006–2008). J. Am. Vet. Med. Assoc. 2010, 237, 1052–1055. [Google Scholar] [CrossRef]
- Keller, E.T.; MacEwen, E.G.; Rosenthal, R.C.; Helfand, S.C.; Fox, L.E. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J. Vet. Intern. Med. 1993, 7, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lori, J.C.; Stein, T.J.; Thamm, D.H. Doxorubicin and cyclophosphamide for the treatment of canine lymphoma: A randomized, placebo-controlled study. Vet. Comp. Oncol. 2010, 8, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, J.W.; Cripps, P.; Marrington, A.M. Epirubicin as part of a multi-agent chemotherapy protocol for canine lymphoma. Vet. Comp. Oncol. 2013, 11, 185–198. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Ernst, T.; Kessler, M.; Lautscham, E. Multicentric lymphoma in 411 dogs—An epidemiological study. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. 2016, 44, 245–251. [Google Scholar]
- Villamil, J.A.; Henry, J.C.; Hahn, W.A. Hormonal and sex impact on the epidemiology of canine lymphoma. J. Cancer Epidemiol. 2009, 2009, 591753. [Google Scholar] [CrossRef]
- Comazzi, S.; Marelli, S.; Cozzi, M. Breed-associated risks for developing canine lymphoma differ among countries: An European canine lymphoma network study. BMC Vet. Res. 2018, 14, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentheroudakis, G.; Goussia, A.; Voulgaris, E.; Nikolaidis, K.; Ioannidou, E.; Papoudou-Bai, A. High levels of topoisomerase IIα protein expression in diffuse large B-cell lymphoma are associated with high proliferation, germinal center immunophenotype, and response to treatment. Leuk. Lymphoma 2010, 51, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Korkolopoulou, P.; Angelopoulou, M.; Siakantari, M. Evaluation of DNA topoisomerase II alpha expression provides independent prognostic information in non-Hodgkin’s lymphomas. Histopathology 2001, 38, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, M. Topoisomerase II alpha—A fundamental prognostic factor in breast carcinoma. Pol. J. Pathol. 2009, 60, 67–75. [Google Scholar]
- Burgess, D.J.; Doles, J.; Zender, L. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 9053–9058. [Google Scholar] [CrossRef] [Green Version]
- Dwarakanath, B.S.; Khaitan, D.; Mathur, R. Inhibitors of topoisomerases as anticancer drugs: Problems and prospects. Indian J. Exp. Biol. 2004, 42, 649–659. [Google Scholar]
- Zijlstra, J.G.; de Jong, S.; de Vries, E.G.; Mulder, N.H. Topoisomerases, new targets in cancer chemotherapy. Med. Oncol. Tumor Pharmacother. 1990, 7, 11–18. [Google Scholar] [CrossRef]




| Antibody (Anti-) | Type and Clone | Manufacturer | Dilution |
|---|---|---|---|
| CD3 | polyclonal, A0452 | Dako, Glostrup, Denmark | 1:300 |
| CD79αc | monoclonal, HM57 | Dako, Glostrup, Denmark | 1:100 |
| Ki67 | monoclonal, MIB-1 | Dako, Glostrup, Denmark | 1:100 |
| TOPIIα | monoclonal, Ki-S1 | Dako, Glostrup, Denmark | 1:200 |
| Parameter | n = 34 | |
|---|---|---|
| Age | Average: 6.6 (median = 6.0, range: 2.5–13) SD ± 2.9 | |
| Sex | Male (neutered male) | 18 (3) |
| Female (spayed female) | 16 (9) | |
| Breed | Mixed-breed | 10 |
| German shepherd | 9 | |
| Golden retriever | 4 | |
| Boxer | 2 | |
| Dog de Bordeaux | 2 | |
| French bulldog | 2 | |
| Miniature schnauzer | 2 | |
| Rottweiler | 2 | |
| Bernese mountain dog | 1 | |
| Clinical stage | III | 20 |
| IV | 14 | |
| Parameter | Group | n | M | Me | Min | Max | Q1 | Q3 | SD | U | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | DLBCL | 34 | 32.85 | 24.5 | 9 | 135 | 17 | 42 | 24.3 | ||
| CB | 28 | 36.82 | 29.5 | 14 | 135 | 18.5 | 44.5 | 25.04 | 11.5 | <0.001 | |
| IB | 6 | 14.33 | 15 | 9 | 18 | 12 | 17 | 3.39 | |||
| Ki67 | DLBCL | 34 | 42.24 | 38.47 | 23.83 | 72.23 | 34.43 | 49.61 | 12.44 | ||
| CB | 28 | 42.43 | 38.12 | 25.87 | 72.23 | 33.82 | 52.06 | 12.82 | 79 | 0.843 | |
| IB | 6 | 41.35 | 41.75 | 23.83 | 58.37 | 36.2 | 46.21 | 11.57 | |||
| Ki67 | TOPIIα | –1.459 * | 0.154 | ||||||||
| expression +/++ | 12 | 38.1 | 37.13 | 23.83 | 46.21 | 35.68 | 43.52 | 6.11 | |||
| expression +++/++++ | 22 | 44.5 | 39 | 25.87 | 72.23 | 32.09 | 56.56 | 14.45 | |||
| MC | TOPIIα | 82.5 | 0.074 | ||||||||
| expression +/++ | 12 | 21.75 | 21.5 | 9 | 35 | 16.5 | 25 | 7.42 | |||
| expression +++/++++ | 22 | 38.91 | 35.5 | 12 | 135 | 17 | 47 | 28.11 | |||
| TOP II Expression Level | DLBCL | |
|---|---|---|
| CB | IB | |
| + | 4 | 2 |
| 14.29% | 33.33% | |
| ++ | 5 | 1 |
| 17.86% | 16.67% | |
| +++ | 10 | 3 |
| 35.71% | 50.00% | |
| ++++ | 9 | 0 |
| 32.14% | 0.00% | |
| Total | 28 | 6 |
| Mean rank | 18.63 | 12.25 |
| Statistical analysis | U = 52.5 p = 0.159 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimiuk, P.; Łopuszyński, W.; Bulak, K.; Brzana, A. Evaluation of the Proliferative Activity of Diffuse Large B-Cell Lymphoma (DLBCL) in Dogs with Respect to Patient Eligibility for Anthracycline-Based Chemotherapy. Animals 2021, 11, 1183. https://doi.org/10.3390/ani11041183
Klimiuk P, Łopuszyński W, Bulak K, Brzana A. Evaluation of the Proliferative Activity of Diffuse Large B-Cell Lymphoma (DLBCL) in Dogs with Respect to Patient Eligibility for Anthracycline-Based Chemotherapy. Animals. 2021; 11(4):1183. https://doi.org/10.3390/ani11041183
Chicago/Turabian StyleKlimiuk, Paweł, Wojciech Łopuszyński, Kamila Bulak, and Adam Brzana. 2021. "Evaluation of the Proliferative Activity of Diffuse Large B-Cell Lymphoma (DLBCL) in Dogs with Respect to Patient Eligibility for Anthracycline-Based Chemotherapy" Animals 11, no. 4: 1183. https://doi.org/10.3390/ani11041183
APA StyleKlimiuk, P., Łopuszyński, W., Bulak, K., & Brzana, A. (2021). Evaluation of the Proliferative Activity of Diffuse Large B-Cell Lymphoma (DLBCL) in Dogs with Respect to Patient Eligibility for Anthracycline-Based Chemotherapy. Animals, 11(4), 1183. https://doi.org/10.3390/ani11041183