Microplastics in Internal Tissues of Companion Animals from Urban Environments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Characterization
2.2. Sample Preparation
2.3. Sample Analysis
2.4. Contamination Control Measures
2.5. Data Analysis and Statistics
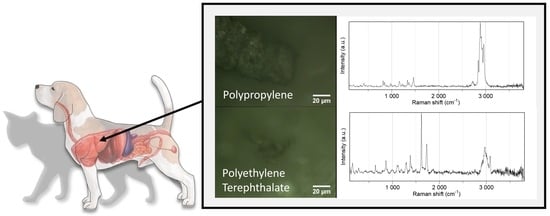
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dris, R.; Gasperi, J.; Tassin, B. Sources and Fate of Microplastics in Urban Areas: A Focus on Paris Megacity. In Freshwater Microplastics. The Handbook of Environmental Chemistry; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 58. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Choi, J. Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2018, 96, 213–220. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One Health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Reguera, P.; Viñas, L.; Gago, J. Microplastics in wild mussels (Mytilus spp.) from the north coast of Spain. Sci. Mar. 2019, 83, 337. [Google Scholar] [CrossRef]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef]
- Volkheimer, G. Persorption of Particles: Physiology and Pharmacology. Adv. Pharmacol. 1977, 14, 163–187. [Google Scholar]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sequeira, I.F.; Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Worldwide contamination of fish with microplastics: A brief global overview. Mar. Pollut. Bull. 2020, 160, 111681. [Google Scholar] [CrossRef] [PubMed]
- Haave, M.; Gomiero, A.; Schönheit, J.; Nilsen, H.; Olsen, A.B. Documentation of Microplastics in Tissues of Wild Coastal Animals. Front. Environ. Sci. 2021, 9, 31. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Polyethylene Terephthalate and Polycarbonate Microplastics in Pet Food and Feces from the United States. Environ. Sci. Technol. 2019, 53, 12035–12042. [Google Scholar] [CrossRef]
- Prata, J.C.; Sequeira, I.F.; Monteiro, S.S.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Preparation of biological samples for microplastic identification by Nile Red. Sci. Total Environ. 2021, 783, 147065. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.V.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Prata, J.C.; Alves, J.R.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Major factors influencing the quantification of Nile Red stained microplastics and improved automatic quantification (MP-VAT 2.0). Sci. Total Environ. 2020, 719, 137498. [Google Scholar] [CrossRef]
- Cowger, W.; Gray, A.; Hapich, H.; Rochman, C.; Lynch, J.; Primpke, S.; Munno, K.; De Frond, H.; Herodotou, O. Open Specy. Available online: www.openspecy.org (accessed on 12 December 2020).
- Pittroff, M.; Müller, Y.K.; Witzig, C.S.; Scheurer, M.; Storck, F.R.; Zumbülte, N. Microplastic analysis in drinking water based on fractionated filtration sampling and Raman microspectroscopy. Environ. Sci. Pollut. Res. 2021, 28, 59439–59451. [Google Scholar] [CrossRef]
- Hayes, G. Gastrointestinal foreign bodies in dogs and cats: A retrospective study of 208 cases. J. Small Anim. Pract. 2009, 50, 576–583. [Google Scholar] [CrossRef]
- Knottenbelt, C.; Mackin, A. Blood transfusions in the dog and cat Part 1. Blood collection techniques. In Pract. 1998, 20, 110–114. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- EFSA. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar]
- FAO. Microplastics in Fisheries and Aquaculture. Available online: https://www.fao.org/3/I7677E/I7677E.pdf (accessed on 1 June 2020).
- Fiorentino, I.; Gualtieri, R.; Barbato, V.; Mollo, V.; Braun, S.; Angrisani, A.; Turano, M.; Furia, M.; Netti, P.A.; Guarnieri, D.; et al. Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Exp. Cell Res. 2015, 330, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, J.H.; Hammond, C.J.; Meulbroek, J.A.; Staas, J.K.; Gilley, R.M.; Tice, T.R. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the peyer’s patches. J. Control. Release 1990, 11, 205–214. [Google Scholar] [CrossRef]
- Hussain, N. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef]
- Volkheimer, G. Hematogeneous dissemination of ingested polyvinyl chloride particles. Ann. N. Y. Acad. Sci. 1975, 246, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Culp, W.T.N.; Aronson, L.R. Splenic foreign body in a cat. J. Feline Med. Surg. 2008, 10, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Frendin, J.; Funkquist, B.; Hansson, K.; Lönnemark, M.; Carlsten, J. Diagnostic imaging of foreign body reactions in dogs with diffuse back pain. J. Small Anim. Pract. 1999, 40, 278–285. [Google Scholar] [CrossRef]
- Hunt, G.B.; Worth, A.; Marchevsky, A. Migration of wooden skewer foreign bodies from the gastrointestinal tract in eight dogs. J. Small Anim. Pract. 2004, 45, 362–367. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lo, P.-Y.; Wu, H.-D.; Chang, C.; Wang, L.-C. Association between indoor air pollution and respiratory disease in companion dogs and cats. J. Vet. Intern. Med. 2018, 32, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Adili, N.; Melizi, M.; Belabbas, H. Species determination using the red blood cells morphometry in domestic animals. Vet. World 2016, 9, 960–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, H.G.; Simmons, G.H.; Deluca, P.P. Distribution of Radiolabeled Subvisible Microspheres after Intravenous Administration to Beagle Dogs. J. Pharm. Sci. 1978, 67, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Blood Clearance and Organ Distribution of Intravenously Administered Polystyrene Microspheres of Different Sizes. J. Bioact. Compat. Polym. 1993, 8, 220–235. [Google Scholar] [CrossRef]
- Herzlinger, G.; Bing, D.; Stein, R.; Cumming, R. Quantitative measurement of C3 activation at polymer surfaces. Blood 1981, 57, 764–770. [Google Scholar] [CrossRef] [Green Version]
- Nissen, O.I.; Galskov, A. Direct Measurement of Superficial and Deep Venous Flow in the Cat Kidney. Circ. Res. 1972, 30, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Slack, J.D.; Kanke, M.; Simmons, G.H.; Deluca, P.P. Acute Hemodynamic Effects and Blood Pool Kinetics of Polystyrene Microspheres following Intravenous Administration. J. Pharm. Sci. 1981, 70, 660–664. [Google Scholar] [CrossRef]
- Ring, G.C.; Blum, A.S.; Kurbatov, T.; Moss, W.G.; Smith, W. Size of microspheres passing through pulmonary circuit in the dog. Am. J. Physiol. Content 1961, 200, 1191–1196. [Google Scholar] [CrossRef]
- Handy, R.D.; Henry, T.B.; Scown, T.M.; Johnston, B.D.; Tyler, C.R. Manufactured nanoparticles: Their uptake and effects on fish—A mechanistic analysis. Ecotoxicology 2008, 17, 396–409. [Google Scholar] [CrossRef]
- Hartenstein, V.; Martinez, P. Phagocytosis in cellular defense and nutrition: A food-centered approach to the evolution of macrophages. Cell Tissue Res. 2019, 377, 527–547. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Porter, C.J.H.; Muir, I.S.; Illum, L.; Davis, S.S. Non-phagocytic uptake of intravenously injected microspheres in rat spleen: Influence of particle size and hydrophilic coating. Biochem. Biophys. Res. Commun. 1991, 177, 861–866. [Google Scholar] [CrossRef]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.-P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope Plastics—The Facts. 2019. Available online: https://www.plasticseurope.org/en/resources/market-data (accessed on 1 June 2020).
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]

| Analysis | Results |
|---|---|
| Independent: Internal Tissue Dependent: Equivalent Diameter | H(4) = 41.411, p < 0.001, n = 617 Ileum—kidney: p = 0.001 Ileum—liver: p = 0.039 Blood clot—ileum: p < 0.001 Blood clot—lungs: p < 0.001 Blood clot—kidney: p = 0.058 |
| Independent: Internal Tissue Dependent: Largest dimension | H(4) = 36.432 p < 0.001, n = 617 Ileum—kidney: p = 0.001 Blood clot—ileum: p < 0.001 Blood clot—lungs: p < 0.001 |
| Independent: Internal Tissue Dependent: Smallest dimension | H(4) = 42.228, p < 0.001, n = 617 Ileum—kidney: p = 0.001 Ileum—liver: p = 0.024 Blood clot—ileum: p < 0.001 Blood clot—lungs: p < 0.001 Blood clot—kidney: p = 0.034 |
| Independent: Internal Tissue Dependent: Circularity | H(4) = 6.375 p = 0.173, n = 617 |
| Independent: Species Dependent: Equivalent Diameter Split: Tissue | Liver: H(1) = 1.494, p = 0.222, n = 63 Lungs: H(1) = 5.383, p = 0.020, n = 118 Kidney: H(1) = 4.458, p = 0.035, n = 152 Ileum: H(1) = 10.475, p = 0.001, n = 126 Blood clot: H(1) = 4.081, p = 0.043, n = 88 |
| Independent: Tissue Dependent: Equivalent Diameter Split: Species | Cat: H(4) = 59.177, p < 0.001, n = 388 Blood clot—lungs: p = 0.009 (10.5 vs. 6.8 µm) Ileum—lungs: p = 0.001 (3.9 vs. 6.8 µm) Ileum—liver: p = 0.001 (3.9 vs. 9.0 µm) Ileum—kidney: p < 0.001 (3.9 vs. 9.6 µm) Ileum—blood clot: p < 0.001 (3.9 vs. 10.5 µm) |
| Dog: H(4) = 4.948, p = 0.293, n = 229 | |
| Independent: Cause of deathDependent: Microplastic concentrationsSplit: Tissue | Liver: H(4) = 9.203, p = 0.056, n = 49 Lungs: H(4) = 2.232, p = 0.693, n = 49 Ileum: H(4) = 1.389, p = 0.846, n = 49 Kidney: H(4) = 3.721, p = 0.445, n = 49 Blood clot: H(4) = 6.846, p = 0.143, n = 46 |
| Independent: Age categories Dependent: Microplastic concentrations Split: Tissue | Liver: H(3) = 4.975, p = 0.174, n = 49 Lungs: H(3) = 0.996, p = 0.802, n = 49 Ileum: H(3) = 3. 815, p = 0.282, n = 49 Kidney: H(3) = 2.087, p = 0.555, n = 49 Blood clot: H(3) = 0.923, p = 0.820, n = 46 |
| Suspected Microplastics in 23% of Sample Filter | ||||||
|---|---|---|---|---|---|---|
| Sizes (µm) | Lungs | Blood Clot | Kidney | Ileum | Liver | |
| ]1, 10] | Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Min–Max | (0.0–21.0) | (0.0–23.0) | (0.0–15.0) | (0.0–34.0) | (0.0–3.0) | |
| Sum | 54 | 26 | 38 | 50 | 8 | |
| ]10, 20] | Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Min–Max | (0.0–11.0) | (0.0–16.0) | (0.0–6.0) | (0.0–4.0) | (0.0–4.0) | |
| Sum | 34 | 23 | 35 | 9 | 16 | |
| ]20, 50] | Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Min–Max | (0.0–3.0) | (0.0–5.0) | (0.0–2.0) | (0.0–2.0) | (0.0–2.0) | |
| Sum | 11 | 10 | 9 | 8 | 6 | |
| ]50, 100] | Median | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Min–Max | (0.0–2.0) | (0.0–1.0) | (0.0–1.0) | (0.0–2.0) | (0.0–0.0) | |
| Sum | 2 | 2 | 4 | 5 | 0 | |
| n | 19 | 8 | 22 | 17 | 14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Carvalho, A.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Microplastics in Internal Tissues of Companion Animals from Urban Environments. Animals 2022, 12, 1979. https://doi.org/10.3390/ani12151979
Prata JC, Silva ALP, da Costa JP, Dias-Pereira P, Carvalho A, Fernandes AJS, da Costa FM, Duarte AC, Rocha-Santos T. Microplastics in Internal Tissues of Companion Animals from Urban Environments. Animals. 2022; 12(15):1979. https://doi.org/10.3390/ani12151979
Chicago/Turabian StylePrata, Joana C., Ana L. Patrício Silva, João P. da Costa, Patrícia Dias-Pereira, Alexandre Carvalho, António José Silva Fernandes, Florinda Mendes da Costa, Armando C. Duarte, and Teresa Rocha-Santos. 2022. "Microplastics in Internal Tissues of Companion Animals from Urban Environments" Animals 12, no. 15: 1979. https://doi.org/10.3390/ani12151979
APA StylePrata, J. C., Silva, A. L. P., da Costa, J. P., Dias-Pereira, P., Carvalho, A., Fernandes, A. J. S., da Costa, F. M., Duarte, A. C., & Rocha-Santos, T. (2022). Microplastics in Internal Tissues of Companion Animals from Urban Environments. Animals, 12(15), 1979. https://doi.org/10.3390/ani12151979