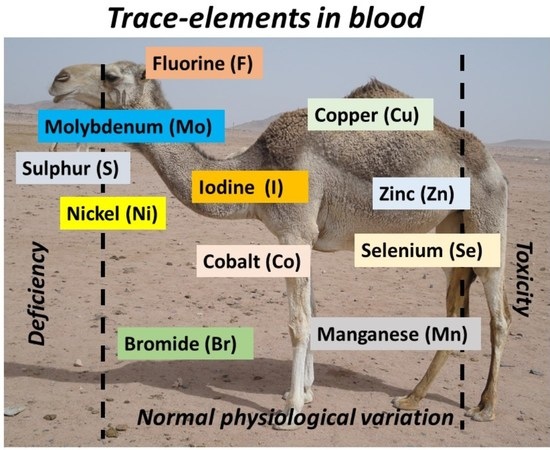
Blood Trace Element Status in Camels: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. General Overview of Trace Minerals Functions
3. Trace Mineral Status in Camel Blood
3.1. Copper
3.1.1. Normal Values
3.1.2. Variation Factors
3.1.3. Copper Deficiency and Toxicity
3.2. Zinc
3.2.1. Normal Values
3.2.2. Variation Factors
3.2.3. Zinc Deficiency and Toxicity
3.3. Iron
3.4. Selenium
3.4.1. Normal Values
3.4.2. Variation Factors
3.4.3. Selenium Deficiency and Toxicity
3.5. Manganese
3.6. Cobalt
3.7. Iodine
3.8. Other Elements
3.8.1. Fluorine
3.8.2. Molybdenum
3.8.3. Sulfur
3.8.4. Bromide
3.8.5. Nickel
4. Functional Indicators of Trace Element Status in Camels
4.1. Ceruloplasmin (Cp)
4.2. Superoxide Dismutase (SOD)
4.3. Glutathione–Peroxidase (GSH-Px)
5. General Discussion
5.1. Limiting Data and Constraints in Camel Trace Element Studies
5.2. Camel Specificity
5.3. Gaps in Knowledge
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Livestock’s Long Shadow. Environmental Issues and Options; LEAD/FAO: Rome, Italy, 2006; Available online: http://www.fao.org/3/a0701e/a0701e.pdf (accessed on 2 February 2021).
- Faye, B. Typology of camel farming system in Saudi Arabia. Emir. J. Food Agric. 2013, 25, 250–260. [Google Scholar] [CrossRef]
- Breulmann, M.; Boer, B.; Wernery, U.; Wernery, R.; El-Shaer, H.; Alhadrami, G.; Gallacher, D.; Peacock, J.; Chaudhary, S.A.; Brown, G. The Camel, from Tradition to Modern Times; Unesco Doha: Doha, Qatar, 2007; Available online: https://fr.scribd.com/document/115925500/The-Camel-From-Tradition-to-Modern-Times (accessed on 2 February 2021).
- Khan, Z.I.; Hussain, A.; Ashraf, M.; Ahraf, Y.; Yousaf, M. A review of mineral Imbalances in grazing livestock and usefulness of soil, dietary components, animal tissues and fluid analysis in the assessment of these imbalances. J. Anim. Vet. Adv. 2004, 3, 394–412. [Google Scholar]
- Khan, Z.I.; Ahraf, M.; Hussain, A.H.W.; Koyro, H.W.; Huchzermeyer, B. Seasonal variation in the status of selenium in goats grazing native and improved pasture in a semiarid region in Pakistan. Dtsch. Tierarztiche Worchenschrift 2005, 11, 460–465. [Google Scholar]
- McDowell, L.R.; Conard, J.H.; Glen, H.F. Minerals for Grazing Ruminants in Tropical Regions. Animal Science Department, Centre for tropical Agricultural, University of Florida; The U.S. Agency for International Development and Caribbean Basin Advisory Group (CBAG): Gainesville, FL, USA, 1993.
- Lacetera, N.; Bernabucci, U.; Ronchi, B.; Nardone, A. Body condition score, metabolic status and milk production of early lactating dairy cows exposed to warm environment. Riv. Agric. Subtrop. Trop. 1996, 90, 43–55. [Google Scholar]
- Ronchi, B.; Bernabucci, U.; Lacetera, N.; Verini Supplizi, A.; Nardone, A. Distinct and common effects of heat stress and restricted feeding on metabolic status of Holstein heifers. Zootec. Nutr. Anim. 1999, 25, 11–20. [Google Scholar]
- Nardone, A.; Lacetera, N.G.; Bernabucci, U.; Ronchi, B. Composition of colostrum from dairy heifers exposed to high air temperatures during late pregnancy and early postpartum period. J. Dairy Sci. 1997, 80, 838–844. [Google Scholar] [CrossRef]
- Moore, C.E.; Kay, J.K.; Collier, R.J.; VanBaale, M.J.; Baumgard, L.H. Effect of supplemental conjugated linoleic acids on heat-stressed brown Swiss and Holstein cows. J. Dairy Sci. 2005, 88, 1732–1740. [Google Scholar] [CrossRef]
- Faye, B. The camel, new challenges for a sustainable development. Trop. Anim. Health Prod. 2016, 48, 689–692. [Google Scholar] [CrossRef]
- Abu Damir, H.; Abbas, T.A.; Ali, M.A. Copper status in breeding and racing camels (Camelus dromedaries) and response to cupric oxide needle capsules. Trop. Anim. Health Prod. 2008, 40, 643–648. [Google Scholar] [CrossRef]
- El-Zubeir, I.E.M.; Ehsan, M.N. Studies on some camel management practices and constraints in pre-urban areas of Khartoum State, Sudan. Int. J. Dairy Sci. 2010, 5, 276–284. [Google Scholar] [CrossRef]
- Faye, B. Camel Farming Sustainability: The Challenges of the Camel Farming System in the XXIth Century. J. Sustain. Dev. 2013, 6, 74–82. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M. Camel Clinical Biochemistry and Hematology. Chapter 7. Trace Elements; Springer: New York, NY, USA, 2018; pp. 217–274. [Google Scholar] [CrossRef]
- Deen, A.; Bhati, A.; Sahani, M. Trace mineral profiles of camels blood and sera. J. Camel Pract. Res. 2004, 11, 135–136. [Google Scholar]
- El-Bahrawy, K.; El Hassanein, E.E. Seasonal variation of some blood and seminal plasma biochemical parameters of male Dromedary camel. Am.-Eurasian J. Agric. Environ. Sci. 2011, 10, 354–360. [Google Scholar]
- Ali, A.; Tharwat, M.; Al-Sobayl, L. Hormonal, biochemical, and hematological profiles in female camels (Camelus dromedaries) affected with reproductive disorders. Anim. Reprod. Sci. 2010, 118, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.J. The incidence of trace element deficiency diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981, 294, 3–8. [Google Scholar] [CrossRef]
- Essawi, W.M.; Gouda, H.F. Inter-relationship between some trace elements during pregnancy and newborn birth weight in dromedary camels. Zagazig Vet. J. 2020, 48, 319–327. [Google Scholar] [CrossRef]
- McDowell, L.R.; Conrad, J.H.; Ellis, G.L.; Loosli, J.K. Minerals for Grazing Ruminants in Tropical Regions; Centre for Tropical Agriculture University of Florida Gainesville and the US Agency for International Development: Gainesville, FL, USA, 1983. [Google Scholar]
- Bicknell, D.V.M. Trace minerals and reproduction. Zimb. Herd Book 1995, 21, 19. [Google Scholar]
- Faye, B.; Bengoumi, M. Trace elements in camels: A review. Biol. Trace Elem. Res. 1994, 42, 1–11. [Google Scholar] [CrossRef]
- Faye, B.; Saint-Martin, G.; Cherrier, R. The influence of high dietary protein, energy, and mineral intake on deficient young camels (Camelus dromedaries). I. Change in mineral status. Comp. Biochem. Physiol. 1992, 102, 417–424. [Google Scholar] [CrossRef]
- Liu, Z.P.; Ma, Z.; Zhang, Y.J. Studies on the relationship between sway disease of Bactrian camels and copper status in Gansu Province. Vet. Res. Commun. 1994, 18, 251–260. [Google Scholar] [CrossRef]
- Seboussi, R.; Faye, B.; Alhadrami, G.; Askar, M.; Bengoumi, M.; Elkhouly, A. Chronic selenosis in camels. J. Camel Pract. Res. 2009, 16, 25–38. [Google Scholar]
- Diacono, E.; Bengoumi, M.; Kessabi, M.; Abdendi, E.; Faye, B. Hydrotelluric and industrial fluorosis survey in the dromedary camel the south of Morocco. In Proceedings of the International Workshop, “Impact of pollution on animal products”, Almaty, Kazakhstan, 27–30 September 2007; Faye, B., Sinyavskiy, Y., Eds.; NATO Series. Springer: Dordrecht, The Netrherlands, 2008; pp. 85–90. [Google Scholar] [CrossRef]
- Mertz, W. The essential trace elements. Sciences 1981, 18, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Mülling, C.H.; Fakler, T.M. Invited review: Formation of keratins in the bovine claw: Roles of hormones, minerals, and vitamins in functional claw integrity. J. Dairy Sci. 2004, 87, 797–809. [Google Scholar] [CrossRef]
- Andrieu, S. Is there a role for organic trace element supplements in transition cow health? Vet. J. 2008, 176, 77–83. [Google Scholar] [CrossRef]
- Healy, J.; Tipton, K. Ceruloplasmin and what it might do. J. Neural Transmis. 2007, 114, 777–781. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Socha, M.T.; DeFrain, J.M. Role of trace minerals in the immune system. In Proceedings of the Penn State Dairy Cattle Nutrition Workshop, Grantville, PA, USA, 22–23 April 2008; pp. 39–52. [Google Scholar]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Predieri, G.; Tegoni, M.; Cinti, E.; Leonardi, G.; Ferruzza, S. Metal chelates of 2-hydroxy-4-methylthiobutanoic acid in Anim. Feeding: Preliminary investigations on stability and bioavailability. J. Inorg. Bioch. 2003, 95, 221–224. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Abdelrahman, M.M.; Rifat, U.K.; Raafat, M.H. Antioxidant status and immune responses of growing camels supplemented a long-acting multi trace minerals rumen bolus. Italian J. Anim. Sci. 2016, 15, 343–349. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kawamura, T.; Shimada, S. Zinc and skin biology. Arch. Biochem. Biophys. 2016, 611, 113–119. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and skin disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef]
- Hay, V.W.; Swenson, M.J. Minerals and bones. In Dukes Physiology of Domestic Animals, 10th ed.; Cornel University Press: Ithaca, NY, USA, 1985; pp. 449–466. [Google Scholar]
- Tinggi, U. Essentiality and toxicity of selenium and its status in Australia: A review. Toxicol. Lett. 2003, 137, 103–110. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.H.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 42319. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.; Erikson, K.M.; Aschner, M. Manganese: Its Role in Disease and Health. Met. Ions Life Sci. 2019, 19, 253–266. [Google Scholar] [CrossRef]
- González-Montaña, J.R.; Escalera-Valente, F.; Alonso, A.J.; Lomillos, J.M.; Robles, R.; Alonso, M.E. Relationship between vitamin B12 and cobalt metabolism in domestic ruminant: An update. Animals 2020, 10, 1855. [Google Scholar] [CrossRef]
- Soriguer, F.; Gutierrez-Repiso, C.; Gonzalez-Romero, S.; Olveira, G.; Garriga, M.J.; Velasco, I.; Santiago, P.; de Escobar, G.M.; Garcia-Fuentes, E. Iodine concentration in cow’s milk and its relation with urinary iodine concentrations in the population. Clin. Nut. 2011, 30, 44–48. [Google Scholar] [CrossRef]
- Miller, W.J. Mineral and Vitamin Nutrition of Dairy Cattle. J. Dairy Sci. 1981, 64, 1196–1206. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm Animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Ronchi, B.; Nardone, A. Markers of oxidative status in plasma and erythrocytes for transition dairy cows during hot season. J. Dairy Sci. 2002, 85, 2173–2179. [Google Scholar] [CrossRef]
- Löhrke, B.; Viergutz, T.; Kanitz, W.; Losand, B.; Weiss, D.G.; Simko, M. Short Communication: Hydroperoxides in Circulating Lipids from Dairy Cows: Implications for Bioactivity of Endogenous-Oxidized Lipids. J. Dairy Sci. 2005, 38, 1708–1710. [Google Scholar] [CrossRef]
- Waldron, M. Metabolic considerations for immunity. In Proceedings of the Mid-South Ruminant Nutrition Conference, Arlington, TX, USA, 25–26 April 2010; pp. 9–18. [Google Scholar]
- Sordillo, L.M.; Contreras, G.A.; Aitken, S.L. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev. 2009, 10, 53–63. [Google Scholar] [CrossRef]
- Bowman, G.; Richards, J.; Vázquez-Añión, M. Improving the antioxidant status of the dairy cow through nutrition. In Proceedings of the Intermountain Nutrition Conference, Salt Lake City, UT, USA, 29–30 January 2008. [Google Scholar]
- Campbell, M.H.; Miller, J.K. Effect of supplemental dietary vitamin E and zinc on reproductive performance of dairy cows and heifers fed excess iron. J. Dairy Sci. 1998, 81, 2693–2699. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Kinacaid, R.L.; Elzubeir, E.A. Mineral Deficiencies in grazing dairy cattle in Kordofan and Darfur regions in western Sudan. Trop. Anim. Health Prod. 1998, 30, 123–135. [Google Scholar] [CrossRef]
- Abdelrahman, M.M. The mineral status of grazing Awassi sheep at Northern part of Jordan. Egypt. J. Anim. Prod. 2003, 40, 55–62. [Google Scholar] [CrossRef]
- Yokus, B.; Cakir, U.D. Seasonal and Physiological Variations in Serum Chemistry and Mineral Concentrations in Cattle. Biol. Trace Element Res. 2006, 109, 255–266. [Google Scholar] [CrossRef]
- White, C.L.; Treacher, T.; Bahhad, F. The vitamin and mineral status of sheep in West Asia. In: Proc. IVth International Symposium on the Nutrition of Herbivores. Ann. Zoot. 1995, 44, 328. [Google Scholar] [CrossRef]
- Al-Attas, S.A. Determination of Essential elements in milk and urine of camel and in Nigella sativa seeds. Arab. J. Camels. 2008, 1, 123–129. [Google Scholar]
- Baba, W.N.; Rasool, N.; Selvamuthukumara, M. A review on nutritional composition, health benefits, and technological interventions for improving consumer acceptability of camel meat: An ethnic food of Middle East. J. Ethn. Food 2021, 8, 18. [Google Scholar] [CrossRef]
- Abu Damir, H. Mineral deficiencies, toxicities and imbalances in the camel (Camelus dromedarius): A review. Vet. Bull. 1998, 68, 1103–1119. [Google Scholar]
- Bengoumi, M.; Faye, B.; El Kasmi, K.; Tressol, J.C. Facteurs de variation des indicateurs plasmatiques du statut nutritionnel en oligo-éléments chez le dromadaire au Maroc. 1. Valeurs usuelles et variations physiologiques. Rev. Elev. Méd. Vét. Pays Trop. 1995, 48, 271–276. [Google Scholar] [CrossRef]
- Bengoumi, M.; Faye, B.; Tressol, J.C.; Bengoumi, D. Facteurs de variation des indicateurs plasmatiques du statut nutritionnel en oligo-éléments chez le dromadaire au Maroc. 2. Effet d’une complémentation minérale. Rev. Elev. Méd. Vét. Pays Trop. 1995, 48, 276–280. [Google Scholar] [CrossRef]
- Elrayah, H.A.; Barri, M.E.S.; Abdelrahman, S.H. Trace elements level in camels (Camelus dromedarius) western Sudan (Kordofan state). J. Camel Pract. Res. 2010, 17, 263–267. [Google Scholar]
- Faye, B.; Grillet, C.; Tessema, A. Teneur en oligo-éléments dans les fourrages et le plasma des ruminants domestiques en Ethiopie. Rev. Elev. Méd. Vét. Pays Trop. 1986, 39, 227–237. [Google Scholar] [CrossRef]
- Faye, B.; Kamil, M.; Labonne, M. Teneur en oligo-éléments dans les fourrages et le plasma des ruminants domestiques en République de Djibouti. Rev. Elev. Méd. Vét. Pays Trop. 1990, 43, 364–373. [Google Scholar] [CrossRef]
- Al-Busadah, K.A. Trace-elements status in camels, cattle and sheep in Saudi Arabia. Pak. J. Biol. Sci. 2003, 6, 1856–1859. [Google Scholar] [CrossRef]
- Seboussi, R.; Faye, B.; Alhadrami, G. Facteurs de variation de quelques éléments trace (sélénium, cuivre, zinc) et d’enzymes témoins de la souffrance musculaire (CPK, ALT et AST) dans le sérum du dromadaire (Camelus dromedarius) aux Emirats Arabes Unis. Rev. Elev. Méd. Vét. Pays Trop. 2004, 57, 87–94. [Google Scholar] [CrossRef]
- Eltahir, Y.E.; Ali, H.M.; Mansur, M.H.; Mahgoub, O. Serum mineral contents of the Omani racing Arabian camel (Camelus dromedaries). J. Anim. Vet. Adv. 2010, 9, 764–770. [Google Scholar] [CrossRef]
- Badiei, K.K.; Mostaghni, K.; Pourjafar, A. Serum and tissue elements in Iranian camels (Camelus dromedarius). Comp. Clin. Pathol. 2006, 15, 103–106. [Google Scholar] [CrossRef]
- Vyas, S.; Saini., N.; Kiradoo., B.D.; Lukha., A.; Kishore., N.; Mal., G.; Pathak, K.M. Biochemical and trace mineral profile in post-parturient dromedary camel (Camelus dromedarius). Indian J. Anim. Sci. 2011, 81, 47–48. [Google Scholar]
- Zongping, L. Studies on the Haematology and Trace Element Status of Adult Bactrian Camels (Camelus bactrianus) in China. Vet. Res. Commun. 2003, 27, 397–405. [Google Scholar] [CrossRef]
- Faye, B. How many large camelids in the world? A synthetic analysis of the world camel demographic changes. Pastor. Res. Pol. Pract. 2020, 10, 25. [Google Scholar] [CrossRef]
- Schillhorn van Veen, T.W.; Loeffler, I.K. Mineral deficiency in ruminants in subsaharan africa: A review. Trop. Anim. Health Prod. 1990, 22, 197–205. [Google Scholar] [CrossRef]
- Laven, R.A.; Lawrence, K.E.; Livesey, C.T. An evaluation R.A. of the copper sequestrated during clotting in cattle: Is it just caeruloplasmin? Vet. J. 2008, 176, 397–399. [Google Scholar] [CrossRef]
- Wernery, U.; Abraham, A.A.; Jyothi, T.; Abubakar, A.Y.; George, R.M. Mineral and vitamin contents in the blood of racing dromedaries in the United Arab Emirates. J. Camel Pract. Res. 2009, 16, 39–40. [Google Scholar]
- Kassilly, F.N. Forage quality and camel feeding patterns in Central Baringo, Kenya. Livest. Prod. Sci. 2002, 78, 175–182. [Google Scholar] [CrossRef]
- Moty, I.A.; Mulla, A.; Zaafer, S.A. Copper, iron and zinc in the serum of Egyptian farm Animals. Sudan Agric. J. 1968, 3, 146–151. [Google Scholar]
- Tartour, G. Copper status in Livest., pasture and soil in Western Sudan. Trop. Anim. Health Prod. 1975, 7, 87–94. [Google Scholar] [CrossRef]
- Abu Damir, H.; Tartour, G.; Adam, S.E.I. Mineral contents in Livest. in eastern Sudan. Trop. Anim. Health Prod. 1983, 15, 15–16. [Google Scholar] [CrossRef]
- Shekhawat, V.S. Some Studies on Serum Trace Mineral (Zinc, Copper and Iron) Levels of Ruminants in Arid Tract of Western Rajasthan. Master’s Thesis, Sukhadia University, Udaipur, India, 1983. [Google Scholar]
- Faye, B.; Grillet, C. La carence en cuivre chez les ruminants domestiques de la région d′Awash. Rev. Elev. Méd. Vét. Pays Trop. 1984, 37, 42–60. [Google Scholar] [CrossRef]
- Faye, B.; Grillet, C.; Tessema, A.; Kamil, M. Copper deficiency in east African Rift Valley. Trop. Anim. Health Prod. 1991, 23, 172–180. [Google Scholar] [CrossRef]
- Faye, B.; Seboussi, R.; Askar, M. Trace elements and heavy metals in healthy camel blood of United Arab Emirates. J. Camel Pract. Res. 2005, 12, 1–6. [Google Scholar]
- Eltohamy, M.M.; Salama, A.; Youssef, A.E.A. Blood constituents in relation to the reproductive state in she-camel (Camelus dromedarius). Beitr. Zür Tropik. Landwirtsch. Vet. Med. 1986, 24, 425–430. [Google Scholar]
- Mohamed, H.E. The zinc and copper content of the plasma of Sudanese camels (Camelus dromedarius). Vet. Res. Commun. 2004, 28, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Kuria, S.G.; Tura, I.A.; Amboga, S.; Walaga, H.K. Status of minerals (Camelus dromedarius) in northeastern Kenya as evaluated from the blood plasma. Livest. Res. Rural Dev. 2013, 25, 1–5. [Google Scholar]
- Patel, A.; Lateef, A.; Haque, N.; Joshi, A.; Patel, P. Physiological status of some serum micro-minerals in kutchi camel during different stages of lactation. Int. J. Livest. Res. 2019, 9, 233–239. [Google Scholar] [CrossRef]
- Faye, B.; Mulato, C. Facteurs de variation des paramètres protéo-énergétiques, enzymatiques et minéraux dans le plasma chez le dromadaire de Djibouti. Rev. Elev. Méd. Vét. Pays Trop. 1991, 44, 325–334. [Google Scholar] [CrossRef]
- Pourjafar, M.; Badiei, K.; Nazifi, S.; Chalmeh, A.; Setayesh, A.; Naghib, M. Correlations between serum trace elements (selenium, copper and zinc) and antioxidant vitamins (vitamin A, E and C) in clinically healthy dromedary camels. J. Fac. Vet. Med. Istanbul Univ. 2014, 40, 7–13. [Google Scholar] [CrossRef]
- Hussein, M.F.; Basmaeil, S.M.; Bakkar, M.N.; Gar-El-Nabi, A.R. Serum levels of some electrolytes and trace elements in camel calves during first year of life. J. Appl. Anim. Res. 1992, 2, 13–18. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Al Jumaah, R.R.; Ayadi, M. Variation of copper, zinc, manganese and magnesium in blood serum and tissues of two breeds of dromedary camels in Saudi Arabia. Asian J. Anim. Vet. Sci. 2013, 8, 91–99. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mutassim, A.; Raafat, H. Effect of long-acting trace mineral rumen bolus supplement on growth performance, metabolic profiles, and trace mineral status of growing camels. Trop. Anim. Health Prod. 2016, 48, 763–768. [Google Scholar] [CrossRef]
- Abdelrahim, A.G. The relationships between the concentrations of serum Cu and Zn and the activities of the serum enzymes copper oxidase and alkaline phosphatase of the dromedary (Camelus dromedarius). J. Arid Environ. 1983, 6, 265–268. [Google Scholar] [CrossRef]
- Kinne, J.; Nagy, P.; Wernery, U. Serum copper levels in dromedaries after long term exogenous copper supplementation. J. Camel Pract. Res. 2003, 10, 121–124. [Google Scholar]
- Osman, N.I.E.D. Effect of copper supplemented salt licks on total and TCA-soluble plasma copper concentrations in Omani camels. J. Camel Pract. Res. 2012, 29, 1–5. [Google Scholar]
- Mohamed, M.H.; Mohamed, A.H.; Locatelli, A. Water deprivation effects on the hematological and hematochemical pictures of Camelus dromedarius. Rev. Elev. Méd. Vét. Pays Trop. 1984, 37, 313–317. [Google Scholar] [CrossRef]
- Ahmed, W.M.; Nada, A.R. Some serum biochemical values of dromedary camels with impaired fertility. Pak. Vet. J. 1993, 13, 16–18. [Google Scholar]
- Al-Dahlimy, A.M.B.; Aldhalemi, A.A.; Aldhalemi, M.A.; Bustani, G.S. Study of the deficiency of some elements and some vital variables in camel’s blood. Plant Arch. 2020, 20, 8945–8949. [Google Scholar]
- Abdel-Saeed, H. Clinical, hematobiochemical and trace-elements alterations in camels with sarcoptic mange (Sarcoptes scabiei var cameli) accompanied by secondary pyoderma. J. Appl. Vet. Sci. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Tuteja, F.C.; Dixit, S.K.; Deen, A.; Bhati, A.; Sahani, M.S. Mineral antioxidant status in serum and its relationship, with somatic cell count in camel milk. J. Camel Pract. Res. 2004, 11, 59–62. [Google Scholar]
- Heidarpour, M.; Mohri, M.; Borji, H.; Moghdass, E. Oxidative stress and trace elements in camel (Camelus dromedarius) with liver cystic echinococcosis. Vet. Parasitol. 2012, 187, 459–463. [Google Scholar] [CrossRef]
- Hassan, H.; Zaghawa, A.; Kamr, A.; Aly, M.; Nayel, M.; Elsify, A.; Salama, A.; Abdelazeim, A. Serum vitamin A and E, copper, zinc and selenium concentrations and their relationship with health outcomes in dromedary hospitalized camels (Camelus dromedarius). Open Vet. J. 2018, 8, 378–385. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ashraf, M.; Ahmad, N.; Amhad, K.; Valeem, E.E. Availability of nutritional minerals (Cobalt, Copper, iron, manganese and Zinc) in pastures of central Punjab for farm Livestock. Pak. J. Bot. 2009, 41, 1603–1609. [Google Scholar]
- Faye, B. Mangrove, sécheresse et dromadaires. Rev. Sécheresse 1993, 4, 47–55. [Google Scholar]
- Gould, L.; Kendall, N.R. Role of the rumen in copper and thiomolybdate absorption. Nut. Res. Rev. 2011, 24, 176–182. [Google Scholar] [CrossRef]
- Abu Damir, H.; Eldirdiri, N.I.; Adam, S.E.I.; Howarth, J.A.; Salih, Y.M.; Idris, O.F. Experimental copper poisoning in the camel (Camelus dromedarius). J. Comp. Pathol. 1993, 108, 191–208. [Google Scholar] [CrossRef]
- Mustafa, A.B.; Sayied, A.A.; Atti, K.A.A. Trace minerals profile in wild pasture and in the blood serum of camel in Butana region. Res. Opin. Anim. Vet. Sci. 2012, 2, 329–333. [Google Scholar]
- Parekar, S.S.; Sanjeev, K.; Mody, S.K.; Kathirvelan, C.; Bhagwat, S.R. Trace mineral bioprofile of male camels. Indian Vet. J. 2009, 86, 1184. [Google Scholar]
- Faye, B.; Bengoumi, M. Comparative trace elements status in camel and cow. J. Camel Pract. Res. 1997, 4, 213–215. [Google Scholar]
- Fahmy, L.S.; Berbish, E.A.; Teleb, H.M.; Hegazy, A.A. Effect of zinc supplementation on wound healing in camels. J. Camel Sci. 2004, 1, 76–80. [Google Scholar]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc Homeostasis in Humans. J. Nut. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Sena, D.S.; Mal, G.; Sahani, M.S.; Bhati, A. Comparative studies on micromineral profile in camels. Indian Vet. J. 2007, 84, 698–700. [Google Scholar]
- Bengoumi, M.; Essamadi, K.; Tressol, J.C.; Faye, B. Comparative study of copper and zinc metabolism in cattle and camel. Biol. Trace Elem. Res. 1998, 63, 81–94. [Google Scholar] [CrossRef]
- Khamis, G.F.; El-Naser, E.M.A.; Aamer, A.A. Assessment of some trace elements in healthy camel, cattle and buffalos. Assiut Vet. Med. J. 2011, 57, 159–169. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M. Le dromadaire face à la sous-nutrition minérale: Un aspect méconnu de son adaptabilité aux conditions désertiques. Rev. Sécheresse 2000, 11, 155–161. [Google Scholar]
- Yu, S.; Beynen, A.C. The combined effect of high iron and zinc intake on copper status in rats. Biol. Trace Elem. Res. 1994, 42, 71–79. [Google Scholar] [CrossRef]
- Desalegn, T.; Mohammed, Y.K.; Shimelis, B. Critical macro and microminerals concentration in the blood serum of camel (Camelus dromedarius) in Jijiga district, easter Ethiopia. Livest. Res. Rural Dev. 2012, 24, 4. [Google Scholar]
- Abdalla, O.M.; Wasfi, I.A.; Gadir, F.A. The Arabian race camel normal parameters. 1. Haemogram, enzymes and minerals. Comp. Biochem. Physiol. 1988, 90, 237–239. [Google Scholar] [CrossRef]
- Kamili, A.; Faye, B.; Bengoumi, M.; Tligui, N.S. Invited review: Camel skin diseases survey in Morocco. J. Camelid. Sci. 2019, 12, 1–16. [Google Scholar]
- Kamili, A.; Faye, B.; Mbesse Kongbonga, Y.; Bengoumi, M.; Tligui, N.S.; Ghalila, H. Determination of zinc in camel skin by Laser Induced Breakdown Spectroscopy. Biol. Trace Elem. Res. 2020, 198, 472–477. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nut. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Diacono, E.; Meldebekova, A.; Konuspayeva, G.; Faye, B. Plant, Water and Milk pollution in Kazakhstan. In Proceedings of the International Workshop, “Impact of pollution on Animal products”, Almaty, Kazakhstan, 27–30 September 2007; Faye, B., Sinyavskiy, Y., Eds.; NATO Series. Springer: Dordrecht, The Netherlands, 2008; pp. 107–116. [Google Scholar] [CrossRef]
- Faye, B.; Seboussi, R. Selenium in camel—A review. Nutrients 2009, 1, 30–49. [Google Scholar] [CrossRef]
- dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of Selenium Deficiency and Toxicity Worldwide: Affected Areas, Selenium-Related Health Issues, and Case Studies; Pilon-Smits, E., Winkel, L., Lin, Z.Q., Eds.; Springer: Cham, Switzerland, 2017; Volume 11. [Google Scholar] [CrossRef]
- Pleban, P.A.; Munyani, A.; Beachum, J. Determination of selenium concentration and glutathione peroxidase activity in plasma and erythrocytes. Clin. Chem. 1982, 28, 311–316. [Google Scholar] [CrossRef]
- Hamliri, A.; Olson, W.G.; Johnson, D.W.; Kessabi, M. Evaluation of biochemical evidence of congenital nutritional myopathy in the two-week prepartum fetuses from selenium-deficient ewes. J. Am. Vet. Med. Assoc. 1990, 51, 1112–1115. [Google Scholar]
- Seboussi, R.; Faye, B.; Alhadrami, G.; Askar, M.; Ibrahim, W.; Hassan, K.; Mahjoub, B. Effect of different selenium supplementation levels on selenium status in camel. Biol. Trace Elem. Res. 2008, 123, 124–138. [Google Scholar] [CrossRef]
- Seboussi, R.; Faye, B.; Askar, M.; Hassan, K.; Alhadrami, G. Effect of selenium supplementation on blood status and milk, urine and fecal excretion in pregnant and lactating camel. Biol. Trace Elem. Res. 2009, 128, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Faye, B.; Saleh, S.; Konuspayeva, G.; Musaad, A.; Bengoumi, M.; Seboussi, R. Comparative effect of organic and inorganic selenium supplementation on selenium status in camel. J. King Saud Univ. Sci. 2014, 26, 149–158. [Google Scholar] [CrossRef]
- Abdelrahim, A.G. The relationship between whole blood selenium (Se) concentration and the activity of the seleno-enzyme, glutathione peroxidase (GSH-Px E.C.I.11.1.9) in camel (Camelus dromedarius). J. Arid Environ. 2005, 62, 359–362. [Google Scholar] [CrossRef]
- Bengoumi, M.; Essamadi, A.K.; Tressol, J.C.; Chacornac, J.P.; Faye, B. Comparative effect of selenium concentration and erythrocyte gluthatione peroxidase activity in cattle and camels. Anim. Sci. 1998, 67, 461–466. [Google Scholar] [CrossRef]
- Nafizi, S.; Mansourian, M.; Nikahval, B.; Razavi, S.M. The relationship between serum level of thyroid hormones, trace elements and antioxidant enzymes in dromedary camel (Camelus dromedarius). Trop. Anim. Health Prod. 2009, 41, 129–134. [Google Scholar] [CrossRef]
- Ma, Z. Studies on Sway Disease of Chinese Bactrian Camels. Epidemiological and Aetiological Aspects; International Foundation for Science Project: Stockholm, Sweden, 1995; p. 17. [Google Scholar]
- Barri, M.E.S.; Al-Sultan, S.I. Studies on selenium and vitamin E status of young Megaheem dromedary camels at Al-Ahsa province. J. Camel Pract. Res. 2007, 14, 51–53. [Google Scholar]
- Żarczyńska, K.; Sobiech, P.; Tinson, A. Influence of selenite triglyceride supplementation on selenium blood status and selected hematological and biochemical parameters in camels (Camelus dromedarius). J. Element. 2020, 24, 1363–1373. [Google Scholar] [CrossRef]
- Faye, B.; Althamma, O.; Musaad, A.; Konuspayeva, G.; Bengoumi, M. Effect of selenium injection in pregnant camels on selenium status of their new-born and milk. Emir. J. Food Agric. 2014, 26, 342–348. [Google Scholar] [CrossRef]
- Faye, B.; Seboussi, R.; Al Hadrami, G. Maternal transfer of selenium by blood and milk in camel. In Proceedings of the 2nd Conference of ISOCARD, Djerba, Tunisia, 12–14 March 2009; p. 126. [Google Scholar]
- Abdelrahman, M.M.; Al Jumaah, R.R.; Ayadi, M. Selenium and Iodine status of two camel breeds (Camelus dromedaries) raise under semi-intensive system in Saudi Arabia. Italian J. Anim. Sci. 2013, 12, 740–746. [Google Scholar] [CrossRef]
- Ozdemir, O.; Ciftçi, M.K.; Hatipoglu, F.; Ortatatli, M.; Yavuz, O.; Kanat, O. Nutritional cardiomyopathy in a young camel (C. dromedarius). Eurasian J. Vet. Sci. 2016, 32, 52–54. [Google Scholar] [CrossRef]
- El-Khouly, A.A.; Abbas, T.A.; Moustafa, T. Myocardial dystrophy in camel calves in the United Arab Emirates (field cases). Emir. J. Food Agric. 2001, 13, 11–17. [Google Scholar] [CrossRef]
- Al-Qarawi, A.A.; Abbas, B.; Haroun, E.M.; Mahmoud, O.M.; Al-Hawas, A. Clinicopathological investigation of Selenium responsive myopathy in young adult camels. J. Camel Pract. Res. 2001, 8, 23–27. [Google Scholar]
- Carvalho, P.R.; Pita, M.C.G.; Loureiro, J.E.; Tanaka, H.R.; Ribeiro, J.C.S. Manganese deficiency in bovines: Connection between manganese metalloenzyme dependent in gestation and congenital defects in newborn calves. Pak. J. Nut. 2010, 9, 488–503. [Google Scholar] [CrossRef]
- Liu, Z.P.; Ma, Z. Studies on trace elements status in Bactrian camels. J. Jiangsu Agric. Coll. 1995, 2, 49–52. [Google Scholar]
- Shukla, M.K.; Siddiquee, G.M.; Latif, A.; Parekar, S.S. Plasma trace mineral concentration of Kutuchi camels. Indian J. Vet. Res. 2009, 18, 28–30. [Google Scholar]
- El Khasmi, A. Contribution à l’étude des protéines sériques et de certains minéraux. Rev. Elev. Méd. Vét. Pays Trop. 1989, 25, 71–80. [Google Scholar] [CrossRef]
- Saini, N.; Singh, N.; Kiradoo, B.D.; Pathak, K.M.L. Comparative biochemical and mineral profile of female Indian dromedaries during breeding season. J. Camel Pract. Res. 2009, 16, 189–193. [Google Scholar]
- Burenbayar, R. Supply of Trace-Elements for Female Camels in Mongolia. VIth Int. Trace-Element Symp; Karl-Marx University: Leipzig, Germany, 1989; Volume 2. [Google Scholar]
- Shen, X.; Li, X. Studies of “emaciation ailment” in the Bactrian camel. Afr. J. Biotechnol. 2010, 9, 8492–8497. [Google Scholar] [CrossRef]
- Meena, D.S.; Singh, A.P.; Mali, M.M.; Bargujar, J.; Dixit, S.K.; Gupta, S.R.; Sharma, T.; Dadhich, H. Macro and micro mineral status in different managemental Practices of camel (Camelus dromedarius) in arid region. Vet. Pract. 2017, 18, 225–227. [Google Scholar]
- Sadan, M.; El-Shafaey, E.; Al-Sobayil, F. Diagnosis and treatment of foreign bodies swallowing syndrome in camels (Camelus dromedarius) with special reference to the role of mineral deficiency. J. Vet. Med. Sci. 2020, 82, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Aumont, G.; Lamand, M.; Tressol, J.C. Iodine nutrition in ewe: Effect of low to high iodine intake on iodine content of biological fluids in pregnant and lactating ewes. Reprod. Nut. Dev. 1989, 29, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Tageldin, M.H.; Sid Ahmed El Sawi, A.; Ibrahim, S.G. Observations on colloid goiter of dromedary camels in the Sudan. Rev. Elev. Méd. Vét. Pays Trop. 1985, 38, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Tageldin, M.H.; Abu Damir, H.; Hussein, M.F. Subclinical nodular goiter associated with Hurthle cell, papillary, and adenomatoid hyperplasic nodules in the dromedary camel in the Sultanate of Oman. Comp. Clin. Pathol. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Antoine-Moussiaux, N.; Faye, B.; Vias, G.F. Tuareg ethnodiagnostic skill of camel diseases in Agadez area (Niger). J. Camel Pract. Res. 2005, 12, 85–93. [Google Scholar] [CrossRef]
- Rejeb, A.; Amara, A.; Rezeigui, H.; Crespeau, F.; Delverdier, M. Etude anatomopathologique et hormonale du goitre chez le dromadaire (Camelus dromedarius) dans le sud tunisien. Rev. Med. Vet. 2012, 163, 242–249. [Google Scholar] [CrossRef]
- Abu Damir, H.; Barri, M.E.S.; Tageldin, M.H.; Idris, O.F. Clinical and subclinical colloid goitre in adult camels (Camelus dromedarius) at Kordofan region of Sudan. Br. Vet. J. 1990, 146, 219–227. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.F.; Osman, A.M. Iodine metabolism in domestic Animals in the Sudan using I131. Endokrinologie 1971, 58, 198–204. [Google Scholar] [CrossRef]
- Abdel-Salaam, A.M.; El-Tahan, A.A.H.; Bakr, A.A. Impact of dietary iodine supplementation on productive and reproductive performance of Maghrebian She-camels. IOSR J. Agric. Vet. Sci. (IOSR-JAVS) 2018, 11, 59–69. [Google Scholar] [CrossRef]
- Etzion, Z.; Alfassi, Z.; Lavi, N.; Yagil, R. Halide concentration in camel plasma in various states of hydration. Biol. Trace Elem. Res. 1987, 12, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Bengoumi, M.; Faye, B. Adaptation du dromadaire à la déshydratation. Rev. Sècheresse 2002, 13, 121–129. [Google Scholar] [CrossRef]
- Kessabi, M.; Assimi, B.; Braun, J.P. The effects of fluoride on Animals and plants in the South Safi zone. Sci. Total Environ. 1984, 38, 63–68. [Google Scholar] [CrossRef]
- Karram, M.H.; Mottelib, A.A.; Nafie, T.H.S.; Sayed, A.S. Clinical and biochemical studies in chronic fluorosis and sulphurosis in camels. Assiut Vet. Med. J. 1989, 21, 165–176. [Google Scholar]
- Laatar, A.; Mrabet, D.; Zakraoui, L. La fluorose en Afrique subsaharienne. Rev. Rhum. 2003, 70, 178–182. [Google Scholar] [CrossRef]
- Choubisa, S.L. Why desert camels are least afflicted with osteo-dental fluorosis? Curr. Sci. 2013, 105, 1671–1672. [Google Scholar]
- Kaushik, V.; Kumar, S.; Sankhla, M.S.; Kumar, R. Fluoride Pollution in Drinking Water and its Adverse Effect on Humans & Animal. J. Seybold Rep. 2020, 15, 1035–1042. [Google Scholar]
- Ali, A.; Derar, D.R.; Abdel-Elmoniem, E.M. Impotentia generandi in male dromedary camels: Heavy metal and trace element profiles and their relations to clinical findings and semen quality. Trop. Anim. Health Prod. 2019, 51, 1167–1172. [Google Scholar] [CrossRef]
- Al-Swailem, A.; Al-Dubaib, M.A.; Al-Ghamdi, G.; Al-Yamani, E.A.; Al-Naeem, A.; Al-Mejali, A.M.; Shehata, M.; Mahmoud, O.M. High Sulphur content of water from deep bore wells as a possible cause of polio-encephalitis in a camel. Bulg. J. Vet. Med. 2009, 1, 265–270. [Google Scholar]
- Jia, J.; Chen, J. Chronic nickel-induced DNA damage and cell death: The protection role of ascorbic acid. Environ. Toxico. Int. J. 2008, 23, 401–406. [Google Scholar] [CrossRef]
- Tao, G.; Geng, C.; Tao, Y.; Jiao, H.; Fan, Y. “Roll disease” of camels in Mongolia. Acta Vet. Zootech. Sin. 1995, 26, 541–544. [Google Scholar]
- Essamadi, K.; Bengoumi, M.; Zaoui, D.; Faye, B.; Bellenchi, C.; Musci, G.; Calabrese, L. Purification and partial characterization of camel (Camelus dromedarius) ceruloplasmin. Comp. Bioch. Physiol. Part B 2002, 131, 509–517. [Google Scholar] [CrossRef]
- Essamadi, A.K.; Bengoumi, M.; Chacormac, J.P.; Faye, B. Relationship between plasma copper concentration and ceruloplasmin activity in camel. Trends Comp. Biochem. Physiol. 1998, 5, 211–220. [Google Scholar]
- Bengoumi, M.; Essamadi, K.; Chacornac, J.P.; Tressol, J.C.; Faye, B. Comparative relationship between copper-zinc plasma concentrations and superoxide dismutase activity in camels and cows. Vet. Res. 1998, 29, 557–565. [Google Scholar] [PubMed]
- Chafik, A.; Essamadi, A.; Çelik, S.Y.; Mavi, A. Purification and biochemical characterization of a novel copper, zinc superoxide dismutase from liver of camel (Camelus dromedarius): An antioxidant enzyme with unique properties. Bioorg. Chem. 2019, 86, 428–436. [Google Scholar] [CrossRef]
- Chafik, A.; Essamadi, A.; Çelik, S.Y.; Solak, K.; Mavi, A. Partial purification and some interesting properties of glutathione peroxidase from liver of camel (Camelus dromedarius). Russ. J. Bioorg. Chem. 2018, 44, 41–51. [Google Scholar] [CrossRef]
- Osman, T.E.A.; Al-Busadah, K.A. Normal concentrations of twenty serum biochemical parameters of she-camels, cows and ewes in Saudi Arabia. Pak. J. Biol. Sci. 2003, 6, 1253–1256. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ashraf, M.; Hussain, A.; McDowell, L.R. Seasonal Variation of Trace Elements in a Semiarid Veld Pasture. Comm. Soil Sci. Plant Anal. 2006, 37, 1471–1483. [Google Scholar] [CrossRef]
- Al-Farudh, A.; Al-Sewailem, M.; Usman, A.R.A. Status of selenium and trace elements in some arid soils cultivated with forage plants: A case study from Saudi Arabia. Int. J. Agric. Biol. 2017, 19, 85–92. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M.; Tressol, J.C. Comparative trace-element excretion in camels and cows. J. Camel Pract. Res. 1999, 6, 19–25. [Google Scholar]
- Laudadio, V.; Tufarelli, V.; Dario, M.; Hammadi, M.; Seddik, M.M.; Lacalandra, G.M.; Dario, C. A survey of chemical and nutritional characteristics of halophytes plants used by camels in Southern Tunisia. Trop. Anim. Health Prod. 2009, 41, 209–215. [Google Scholar] [CrossRef] [PubMed]

| Country | Camel | Cattle | Sheep | Goat | Substrat | References |
|---|---|---|---|---|---|---|
| Egypt | 83 | 64 | 82 | NA | Serum | Moty et al. [75] |
| Sudan | 95.3 | 73.8 | 85 | 78.9 | Serum | Tartour [76] |
| Sudan | 92.6 | 86.2 | 94.5 | NA | Serum | AbuDamir et al. [77] |
| India | 94.3 | 86.8 | 88.3 | NA | Serum | Shekhawat [78] |
| Ethiopia | 45 | 37.2 | 24.7 | 41.8 | Plasma | Faye and Grillet [79] |
| Ethiopia | 107 | 64.5 | 95.1 | 89.2 | Plasma | Faye et al. [80] |
| Djibouti | 60.7 | 73.8 | 87.2 | 94.5 | Plasma | Faye et al. [81] |
| Saudi Arabia | 113.5 | 70.2 | 95.6 | NA | Serum | Al-Busadah [64] |
| Country | Camel | Cattle | Sheep | Goat | Substrate | Reference |
|---|---|---|---|---|---|---|
| Egypt | 135 | 144 | 160 | NA | Serum | Moty et al. [75] |
| India | 85.4 | 86.8 | 94.8 | NA | Serum | Shekhawat [78] |
| Ethiopia | 100.4 | 113.5 | 114.2 | 107.7 | Plasma | Faye et al. [62] |
| Djibouti | 46.2 | 97.6 | 71.5 | 65.6 | Plasma | Faye et al. [81] |
| Morocco | 38 | 83 | NA | NA | Plasma | Bengoumi et al. [111] |
| Saudi Arabia | 103.4 | 98.5 | 110.7 | NA | Serum | Al-Busadah [64] |
| Egypt | 104.4 | 96.7 | NA | NA | Serum | Khamis et al. [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, M.M.; Alhidary, I.A.; Aljumaah, R.S.; Faye, B. Blood Trace Element Status in Camels: A Review. Animals 2022, 12, 2116. https://doi.org/10.3390/ani12162116
Abdelrahman MM, Alhidary IA, Aljumaah RS, Faye B. Blood Trace Element Status in Camels: A Review. Animals. 2022; 12(16):2116. https://doi.org/10.3390/ani12162116
Chicago/Turabian StyleAbdelrahman, Mutassim M., Ibrahim A. Alhidary, Riyadh S. Aljumaah, and Bernard Faye. 2022. "Blood Trace Element Status in Camels: A Review" Animals 12, no. 16: 2116. https://doi.org/10.3390/ani12162116
APA StyleAbdelrahman, M. M., Alhidary, I. A., Aljumaah, R. S., & Faye, B. (2022). Blood Trace Element Status in Camels: A Review. Animals, 12(16), 2116. https://doi.org/10.3390/ani12162116