A Possible Mechanism for Double-Yolked Eggs in the Early Stage of Egg-Laying in Zhedong White Goose–Function of IGF1 and LHR Signaling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals and Experimental Procedure
2.3. RNA Isolation, cDNA Synthesis, and Real-Time PCR
2.4. IGF1 Concentration Measurements
2.5. Statistical Analysis
3. Results
3.1. Laying Rate during the First Clutch of Egg-Laying
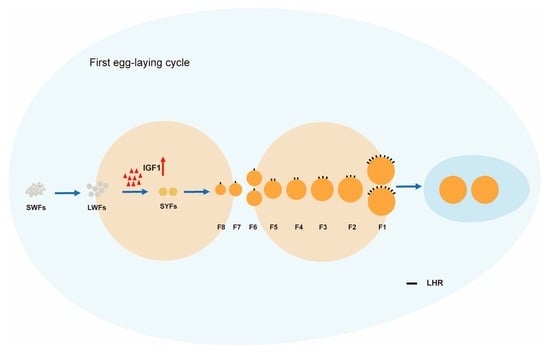
3.2. Ovarian Appearance and Diameter
3.3. Relationship between Expression Levels of LHR and StAR and Follicle Diameter
3.4. IGF1-Related Gene Expression and IGF1 Concentration in the Blood
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolc, A.; Arango, J.; Settar, P.; O’Sullivan, N.P.; Olori, V.E.; White, I.M.S.; Hill, W.G.; Dekkers, J.C.M. Genetic parameters of egg defects and egg quality in layer chickens. Poult. Sci. 2012, 91, 1292–1298. [Google Scholar] [CrossRef]
- Intarakumthornchai, T.; Kesvarakul, R. Double yolk eggs detection using fuzzy logic. PLoS ONE 2020, 15, e0241888. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R.M.; Warren, D.C. The Production of Double Yolked Eggs in the Fowl. Poult. Sci. 1940, 19, 9–17. [Google Scholar] [CrossRef]
- Christmas, R.B.; Harms, R.H. Incidence of double yolked eggs in the initial stages of lay as affected by strain and season of the year. Poult. Sci. 1981, 61, 1290–1292. [Google Scholar] [CrossRef]
- Bruggeman, V.; Onagbesan, O.; Ragot, O.; Metayer, S.; Cassy, S.; Favreau, F.; Jego, Y.; Trevidy, J.J.; Tona, K.; Williams, J.; et al. Feed allowance-genotype interactions in broiler breeder hens. Poult. Sci. 2005, 84, 298–306. [Google Scholar] [CrossRef]
- Francoeur, L.; Stephens, C.S.; Johnson, P.A. Ad Libitum Feeding in Broiler Breeder Hens Alters the Transcriptome of Granulosa Cells of Pre-Hierarchal Follicles. Animals 2021, 11, 2706. [Google Scholar] [CrossRef]
- Johnston, S.A.; Gous, R.M. Extent of variation within a laying flock: Attainment of sexual maturity, double-yolked and soft-shelled eggs, sequence lengths and consistency of lay. Br. Poult. Sci. 2007, 48, 609–616. [Google Scholar] [CrossRef]
- Pan, Y.E.; Liu, Z.C.; Chang, C.J.; Huang, Y.F.; Lai, C.Y.; Walzem, R.L.; Chen, S.E. Feed restriction ameliorates metabolic dysregulation and improves reproductive performance of meat-type country chickens. Anim. Reprod. Sci. 2014, 151, 229–236. [Google Scholar] [CrossRef]
- Walzem, R.L.; Chen, S.E. Obesity-induced dysfunctions in female reproduction: Lessons from birds and mammals. Adv. Nutr. Int. Rev. J. 2014, 5, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Renema, R.A.; Robinson, F.E.; Newcombe, M.; McKay, R.I. Effects of body weight and feed allocation during sexual maturation in broiler breeder hens. 1. Growth and carcass characteristics. Poult. Sci. 1999, 78, 619–628. [Google Scholar] [CrossRef]
- Burke, W.H.; Henry, M.H.; Elezaj, I. Comparison of embryos and chicks that developed as single individuals in double yolk eggs with those that developed in single yolk eggs. Poult. Sci. 1997, 76, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, K.; Tu, K.; Pan, L.; Zhang, W. Identification of double-yolked duck egg using computer vision. PLoS ONE 2017, 12, e0190054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navara, K.J.; Wrobel, E.R. Frequent double ovipositions in two flocks of laying hens. Poult. Sci. 2019, 98, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Salamon, A.; Kent, J.P. Yolk size and ovulation order determine fertility within double-yolked duck (Anas platyrhynchos domesticus) eggs. Reprod. Fertil. Dev. 2016, 28, 440–445. [Google Scholar] [CrossRef]
- Martinat, N.; Crepieux, P.; Reiter, E.; Guillou, F. Extracellular signal-regulated kinases (ERK) 1, 2 are required for luteinizing hormone (LH)-induced steroidogenesis in primary Leydig cells and control steroidogenic acute regulatory (StAR) expression. Reprod. Nutr. Dev. 2005, 45, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Shoham, Z.; Schachter, M.; Loumaye, E.; Weissman, A.; MacNamee, M.; Insler, V. The luteinizing hormone surge—The final stage in ovulation induction: Modern aspects of ovulation triggering. Fertil. Steril. 1995, 64, 237–251. [Google Scholar]
- Thibault, C. Ovulation. Contracept. Fertil. Sex. 1999, 27, 605–613. [Google Scholar] [CrossRef]
- Lei, M. Transcriptome analysis to unravel the gene expression profile of ovarian follicular development in Magang goose. J. Reprod. Dev. 2020, 66, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.C.; Fu, Q.G.; Zhao, R.Q.; Ni, Y.D.; Chen, J. Expression of mRNAs for GHR, IGF-IR, FSHR and LHR in granulosa and theca layers of ovarian follicles of Shaoxing ducks. Acta Genet. Sin. 2003, 30, 840–846. [Google Scholar]
- Johnson, A.L.; A Johnson, P.; Van Tienhoven, A. Ovulatory response, and plasma concentrations of luteinizing hormone and progesterone following administration of synthetic mammalian or chicken luteinizing hormone-releasing hormone relative to the first or second ovulation in the sequence of the domestic hen. Biol. Reprod. 1984, 31, 646–655. [Google Scholar]
- Liu, H.K.; Lilburn, M.S.; Koyyeri, B.; Anderson, J.W.; Bacon, W.L. Preovulatory surge patterns of luteinizing hormone, progesterone, and estradiol-17beta in broiler breeder hens fed ad libitum or restricted fed. Poult. Sci. 2004, 83, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Dentin, R.; Girard, J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004, 30, 398–408. [Google Scholar] [CrossRef]
- Adamek, A.; Kasprzak, A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.S.; Hill-Ricciuti, A.; Francoeur, L.; Johnson, P.A. Feeding level is associated with altered liver transcriptome and follicle selection in hendagger. Biol. Reprod. 2022, 106, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Crochet, S.; Tesseraud, S.; Dupont, J. IGF-1 receptor signaling pathways and effects of AMPK activation on IGF-1-induced progesterone secretion in hen granulosa cells. Domest. Anim. Endocrinol. 2008, 34, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Kusakabe, M.; Swanson, P.; Young, G. Regulation of sex steroid production and mRNAs encoding gonadotropin receptors and steroidogenic proteins by gonadotropins, cyclic AMP and insulin-like growth factor-I in ovarian follicles of rainbow trout (Oncorhynchus mykiss) at two stages of vitellogenesis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 201, 132–140. [Google Scholar]
- Ahumada-Solorzano, S.M.; Martínez-Moreno, C.G.; Carranza, M.; Avila-Mendoza, J.; Luna-Acosta, J.L.; Harvey, S.; Luna, M.; Aramburo, C. Autocrine/paracrine proliferative effect of ovarian GH and IGF-I in chicken granulosa cell cultures. Gen. Comp. Endocrinol. 2016, 234, 47–56. [Google Scholar] [CrossRef]
- Kadakia, R.; Arraztoa, J.A.; Bondy, C.; Zhou, J. Granulosa cell proliferation is impaired in the Igf1 null ovary. Growth Horm. IGF Res. 2001, 11, 220–224. [Google Scholar] [CrossRef]
- Baker, J.; Hardy, M.P.; Zhou, J.; Bondy, C.; Lupu, F.; Bellve, A.R.; Efstratiadis, A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar]
- Zhou, J.; TKumar, R.; Matzuk, M.M.; Bondy, C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol. Endocrinol. 1997, 11, 1924–1933. [Google Scholar] [CrossRef]







| Primers Name | GeneBank Accession | Sequences (5′-3′) | PCR Products (bp) |
|---|---|---|---|
| LHR | NM_204936.1 | F:CGGATACACAACGATGCCCT | 74 |
| R:GACTCCAGTGCCGTTGAAGA | |||
| StAR | KF958133.1 | F:GGAGCAGATGGGAGACTGGA | 91 |
| R:CGCCTTCTCGTGGGTGAT | |||
| IGF1R | XM_013181823.1 | F:CATGTGGTTCGGTTGCTTGG | 198 |
| R:GAGGTATGCCATCCCGTCAG | |||
| IGFBP1 | XM_013197496.1 | F:GCTGTGTGCTGGTGTGTCTA | 83 |
| R:TGTTGGCATTCAGGGTCTCC | |||
| IGFBP3 | XM_013197497 | F:ATGTCTTGAGTCCCAGGGGT | 89 |
| R:TCGACCTTTGGATGGACGAC | |||
| γ-DH | NM_204305.1 | F:GCCATCACAGCCACACAGA | 120 |
| R:TTTCCCCACAGCCTTAGCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, X.; Dai, Z.; Yang, P.; Chen, R.; Guo, B.; Lei, M.; Shi, Z. A Possible Mechanism for Double-Yolked Eggs in the Early Stage of Egg-Laying in Zhedong White Goose–Function of IGF1 and LHR Signaling. Animals 2022, 12, 2964. https://doi.org/10.3390/ani12212964
Liu J, Zhao X, Dai Z, Yang P, Chen R, Guo B, Lei M, Shi Z. A Possible Mechanism for Double-Yolked Eggs in the Early Stage of Egg-Laying in Zhedong White Goose–Function of IGF1 and LHR Signaling. Animals. 2022; 12(21):2964. https://doi.org/10.3390/ani12212964
Chicago/Turabian StyleLiu, Jie, Xingfei Zhao, Zichun Dai, Pengxia Yang, Rong Chen, Binbin Guo, Mingming Lei, and Zhendan Shi. 2022. "A Possible Mechanism for Double-Yolked Eggs in the Early Stage of Egg-Laying in Zhedong White Goose–Function of IGF1 and LHR Signaling" Animals 12, no. 21: 2964. https://doi.org/10.3390/ani12212964
APA StyleLiu, J., Zhao, X., Dai, Z., Yang, P., Chen, R., Guo, B., Lei, M., & Shi, Z. (2022). A Possible Mechanism for Double-Yolked Eggs in the Early Stage of Egg-Laying in Zhedong White Goose–Function of IGF1 and LHR Signaling. Animals, 12(21), 2964. https://doi.org/10.3390/ani12212964