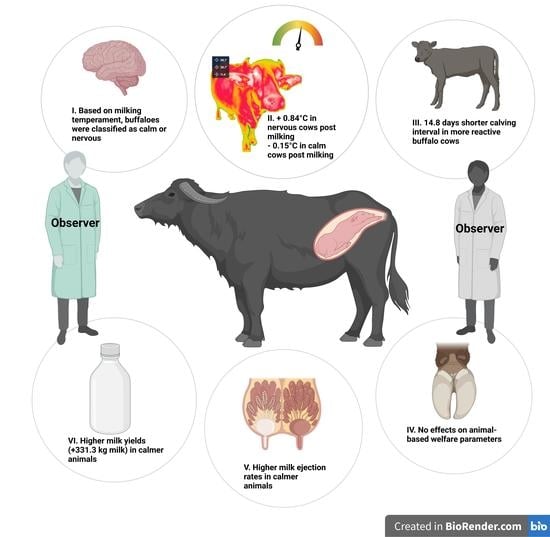
Water Buffalo Responsiveness during Milking: Implications for Production Outputs, Reproduction Fitness, and Animal Welfare
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Temperament Assessment
- The buffalo cow is ruminating, relaxed and extremely calm, no movement;
- The buffalo cow is alert but calm, with occasional head and ear movements;
- The buffalo cow is alert and reactive to the milking machine being put on and taken down, with moderately movements of hind legs;
- The buffalo cow kicks and pendulates her gate from one hind leg to another, defecates, and/or urinates, with abrupt episodic movements;
- The buffalo cow kicks and tries to take the milking machine down, is obviously restless, emits vocalizations, and defecates/urinates, with permanent episodic movements, head butting aggressively.
2.2. Data Collection and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Freslon, I.; Peralta, J.M.; Strappini, A.C.; Monti, G. Understanding Allogrooming Through a Dynamic Social Network Approach: An Example in a Group of Dairy Cows. Front. Vet. Sci. 2020, 7, 535. [Google Scholar] [CrossRef]
- Marino, L.; Allen, K. The psychology of cows. Anim. Behav. Cogn. 2017, 4, 474–498. [Google Scholar] [CrossRef] [Green Version]
- Caicoya, A.L.; Colell, M.; Ensenyat, C.; Amici, F. Problem solving in European bison (Bison bonasus): Two experimental approaches. R. Soc. Open Sci. 2021, 28, 201901. [Google Scholar] [CrossRef]
- Cooke, S. The Ethics of Touch and the Importance of Nonhuman Relationships in Animal Agriculture. J. Agric. Environ. Ethics 2021, 34, 12. [Google Scholar] [CrossRef]
- Simitzis, P.; Tzanidakis, C.; Tzamaloukas, O.; Sossidou, E. Contribution of Precision Livestock Farming Systems to the Improvement of Welfare Status and Productivity of Dairy Animals. Dairy 2022, 3, 12–28. [Google Scholar] [CrossRef]
- Hedlund, L.; Lovlie, H. Personality and production: Nervous cows produce less milk. J. Dairy Sci. 2015, 98, 5819–5828. [Google Scholar] [CrossRef]
- Thomas, C.S.; Bruckmaier, R.M.; Ostensson, K.; Svennersten-Sjaunja, K. Effect of different milking routines on milking-related release of the hormones oxytocin, prolactin and cortisol, and on milk yield and milking performance in Murrah buffaloes. J. Dairy Res. 2005, 72, 10–18. [Google Scholar] [CrossRef]
- Bidarimath, M.; Aggarwal, A. Studies on cisternal and alveolar fractions and its composition and mammary health of Murrah buffaloes administered oxytocin. Trop. Anim. Health Prod. 2007, 39, 433–438. [Google Scholar] [CrossRef]
- Costa, A.; De Marchi, M.; Visentin, G.; Campagna, M.C.; Borghese, A.; Boselli, C. The Effect of Pre-milking Stimulation on Teat Morphological Parameters and Milk Traits in the Italian Water Buffalo. Front. Vet. Sci. 2020, 8, 572422. [Google Scholar] [CrossRef]
- Haskell, M.J.; Simm, G.; Turner, S.P. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 2014, 5, 368. [Google Scholar] [CrossRef]
- Borghese, A.; Rasmussen, M.; Thomas, C.S. Milking management of dairy buffalo. Italian J. Anim. Sci. 2007, 6, 39–50. [Google Scholar] [CrossRef]
- Prasad, R.M.V.; Laxmi, P.J. Studies on the temperament of Murrah buffaloes with various udder and teat shapes and its effect on milk yield. Buffalo Bull. 2014, 33, 170–176. [Google Scholar]
- de Carvalhal, M.V.L.; Sant Anna, A.C.; Pascoa, A.G.; Jung, J.; da Costa, M.J.R.P. The relationship between water buffalo cow temperament and milk yield and quality traits. Livest. Sci. 2017, 198, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, F.; Serrapica, F.; Braghieri, A.; Masucci, F.; Sabia, E.; De Rosa, G. Human-Animal Interactions in Dairy Buffalo Farms. Animals 2019, 16, 246. [Google Scholar] [CrossRef] [Green Version]
- Shahid, R.; Qureshi, Z.I.; Rahman, Z. Hormonal effects on health biomarkers and reproductive performance in buffaloes (Bubalus bubalis) with low, medium and high milk production. J. Anim. Plant Sci. 2021, 31, 960–965. [Google Scholar]
- Mustafa, M.Y.; Saleem, K.; Munir, R.; Butt, T.M. Effect of oxytocin on the productive and reproductive performance of buffalo and cattle in Sheikhupura-Pakistan (A field study). Livest. Res. Rural Dev. 2008, 20, 193. [Google Scholar]
- Faraz, A.; Waheed, A.; Nazir, M.M.; Hameed, A.; Tauqir, N.A.; Mirza, R.H.; Ishaq, H.M.; Bilal, R.M. Impact of oxytocin administration on milk quality, reproductive performance and residual effects in dairy animals—A review. Punjab Univ. J. Zool. 2020, 35, 61–67. [Google Scholar] [CrossRef]
- Orihuela, A.; Mota-Rojas, D.; Strappini, A.; Serrapica, F.; Braghieri, A.; Mora-Medina, P.; Napolitano, F. Neurophysiological Mechanisms of Cow–Calf Bonding in Buffalo and Other Farm Animals. Animals 2021, 11, 1968. [Google Scholar] [CrossRef]
- Byrne, T.J.; Santos, B.F.S.; Amer, P.R.; Martin-Collado, D.; Pryce, J.E.; Axford, M. New breeding objectives and selection indices for the Australian dairy industry. J. Dairy Sci. 2016, 99, 8146–8167. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Brito, L.F.; Alvarenga, A.B.; Wang, Y. Incorporating temperament traits in dairy cattle breeding programs: Challenges and opportunities in the phenomics era. Anim. Front. 2020, 10, 29–36. [Google Scholar] [CrossRef]
- Sewalem, A.; Miglior, F.; Kistemaker, G.J. Short communication: Genetic parameters of milking temperament and milking speed in Canadian Holstein. J. Dairy Sci. 2011, 94, 512–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota-Rojas, D.; Pereira, A.M.F.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Avalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- International Committee for Animal Recording (ICAR) Guidelines, Version 2012, ICAR Section 17 Guidelines for Buffalo Milk Recording. Available online: https://www.icar.org/Guidelines/17-Buffalo-Milk-Recording.pdf (accessed on 30 September 2022).
- Negretti, P.; Bianconi, G.; Bartocci, S.; Terramoccia, S.; Verna, M. Determination of live weight and body condition score in lactating Mediterranean buffalo by Visual Image Analysis. Livest. Sci. 2008, 113, 1–7. [Google Scholar] [CrossRef]
- Welfare Quality®. Welfare Quality Assessment Protocol for Cattle; Welfare Quality Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Webster, A.J.F. Assessment of the welfare of dairy caftle using animal-based measurements: Direct observations and investigation of farm records. Vet. Rec. 2003, 153, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Erdem, H.; Okuyucu, I.C.; Abaci, S.H. Milking temperament of Anatolian buffaloes during early lactation. Appl. Anim. Behav. Sci. 2022, 253, 105679. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Dixit, V.B.; Sethi, R.K.; Khanna, S. Association of breed characteristics with milk production in Murrah buffaloes. Indian J. Anim. Sci. 2007, 77, 1011–1016. [Google Scholar]
- Saltalamacchia, F.; Tripladi, C.; Castellano, A.; Napolitano, F.; Musto, M.; De Rosa, G. Human and animal behaviour in dairy buffalo at milking. Anim. Welf. 2007, 16, 139–142. [Google Scholar]
- Ozenc, E.; Bozkurt, M.F.; Yazici, E.; Seker, E.; Bayraktaroglu, A.G.; Ozcinar, U.; Dogan, N. Teat characteristics in relation to animal temperament during milking in buffaloes, and comparison of buffalo and cow teat morphology. Reprod. Dom. Anim. 2020, 55, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Moore-Foster, R.; Norby, B.; Schewe, R.L.; Thomson, R.; Bartlett, P.C.; Erskine, R.J. Herd-level variables associated with delayed milk ejection in Michigan dairy herds. J. Dairy Sci. 2019, 102, 696–705. [Google Scholar] [CrossRef] [Green Version]
- de la Cruz-Cruz, L.A.; Guerrero-Legarreta, I.; Ramirez-Necoechea, R.; Roldan-Santiago, P.; Mora-Medina, P.; Hernandez-Gonzalez, R.; Mota-Rojas, D. The behaviour and productivity of water buffalo in different breeding systems: A review. Vet. Med. 2014, 59, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, F.; Braghieri, A.; Bragaglio, A.; Rodríguez González, D.; Mora-Medina, P.; Ghezzi, M.D.; Álvarez-Macías, A.; Lendez, P.A.; Sabia, E.; DomínguezOliva, A.; et al. Neurophysiology of Milk Ejection and Prestimulation in Dairy Buffaloes. Animals 2022, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.A.; Dias, M.; Machado, T.M.M. Reproductive and productive performance of water buffaloes in central plateau of Brazil. Italian J. Anim. Sci. 2007, 6, 640–642. [Google Scholar] [CrossRef]
- Parlato, E.; Zicarelli, L. Effect of Calving Interval on Milk Yield in Italian Buffalo Population. J. Buffalo Sci. 2016, 5, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Nava-Trujillo, H.; Valeris-Chacin, R.; Quintero-Moreno, A.; Escalona-Munoz, J. Milk yield at first lactation, parity, and season of calving affect the reproductive performance of water buffalo cows. Anim. Prod. Sci. 2020, 60, 1073–1080. [Google Scholar] [CrossRef]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-Invasive Physiological Indicators of Heat Stress in Cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Laurijs, K.A.; Briefer, E.F.; Reimert, I.; Webb, L.E. Vocalisations in farm animals: A step towards positive welfare assessment. Appl. Anim. Behav. Sci. 2021, 236, 105264. [Google Scholar] [CrossRef]


| Table 100. | Milk Yield (kg/100 DIM) | Milking Speed (kg/min) | Calving Interval (days) | Age at First Calving (months) |
|---|---|---|---|---|
| Cohort | 828.8 ± 57.8 | 0.48 ± 0.044 | 499.2 ± 41.8 | 52.76 ± 2.75 |
| Calm | 924.3 ± 63.6 a | 0.58 ± 0.053 a | 503.9 ± 50.5 | 51.12 ± 2.96 |
| Nervous | 593.0 ± 108.0 b | 0.26 ± 0.047 b | 489.1 ± 76.5 | 56.50 ± 6.04 |
| Significance | p = 0.0082 | p = 0.0015 | p = 0.8962 | p = 0.6092 |
| Temperament | Body Condition Score (BCS) % | Ocular Discharges % | Skin Lesions % | Nasal Discharges % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Welfare Quality® scale | Thin (1–3) | Average (4–6) | Fat (7–9) | 0 | 1 | 0 | 1 | 0 | 1 |
| Cohort | 41.66 | 35.00 | 23.33 | 91.66 | 8.33 | 95.00 | 5.00 | 95.00 | 5.00 |
| Calm | 33.33 | 38.09 | 28.57 | 92.85 | 7.14 | 97.61 | 2.38 | 95.23 | 4.76 |
| Nervous | 61.11 | 27.77 | 11.11 | 88.88 | 11.11 | 88.88 | 11.11 | 94.44 | 5.55 |
| Significance | NS, p = 0.1128 | NS, p = 0.6102 | NS, p = 0.1550 | NS, p = 0.8971 | |||||
| Temperament | Tarsal Joint Lesions % | Hairless Patches % | Cleanliness of | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rump % | Udder % | Hind Legs % | |||||||||
| Welfare Quality® | 0 | 1 | 2 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 2 |
| Cohort | 90.00 | 5.00 | 5.00 | 31.66 | 68.33 | 71.66 | 28.33 | 88.33 | 11.66 | 45.00 | 55.00 |
| Calm | 92.85 | 4.76 | 2.38 | 30.95 | 69.04 | 66.66 | 33.33 | 90.47 | 9.52 | 45.23 | 54.76 |
| Nervous | 83.33 | 5.55 | 11.11 | 33.33 | 66.66 | 83.33 | 16.66 | 83.33 | 16.66 | 44.44 | 55.55 |
| Significance | NS, p = 0.2597 | NS, p = 0.8558 | NS, p = 0.1892 | NS, p = 0.4296 | NS, p = 0.9548 | ||||||
| Source of Variation | Degrees of Freedom DF | Mean Squares MS | F | p-Value | |
|---|---|---|---|---|---|
| Milk yield | A | a − 1 = 1 | 1,380,071.4286 | 7.5985 | 0.0080 |
| B | b − 1 = 2 | 26,968.6667 | 0.1485 | 0.8624 | |
| AB | (a − 1) (b − 1) = 2 | 122,175.4892 | 0.6727 | 0.5146 | |
| Error (residual) | n − ab = 54 | 181,624.8196 | |||
| Calving interval | A | a − 1 = 1 | 2717.6284 | 0.0298 | 0.8637 |
| B | b − 1 = 2 | 227,151.5682 | 2.4897 | 0.0930 | |
| AB | (a − 1) (b − 1) = 2 | 231,094,2865 | 2.5329 | 0.0894 | |
| Error (residual) | n − ab = 51 | 91,237.5818 | |||
| Age at first calving | A | a − 1 = 1 | 361.7877 | 0.8547 | 0.3594 |
| B | b − 1 = 2 | 1413.2408 | 3.3385 | 0.0431 | |
| AB | (a − 1) (b − 1) = 2 | 140.3463 | 0.3315 | 0.7193 | |
| Error (residual) | n − ab = 53 | 423.3154 |
| Temperament | Nasal IRT Temperature (°C) | Orbital IRT Temperature (°C) |  | ||
| Pre-Milking | Post-Milking | Pre-Milking | Post-Milking | ||
| Cohort | 29.33 ± 0.296 | 29.47 ± 0.392 | 31.75 ± 0.192 | 31.74 ± 0.422 | |
| Calm | 29.46 ± 0.305 | 29.31 ± 0.532 | 31.76 ± 0.263 | 31.61 ± 0.591 | |
| Nervous | 29.02 ± 0.734 | 29.86 ± 0.420 | 31.74 ± 0.209 | 32.06 ± 0.289 | |
| Significance | NS, p = 0.916 | NS, p = 0.712 | NS, p = 0.958 | NS, p = 0.958 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mincu, M.; Gavojdian, D.; Nicolae, I.; Olteanu, A.C.; Bota, A.; Vlagioiu, C. Water Buffalo Responsiveness during Milking: Implications for Production Outputs, Reproduction Fitness, and Animal Welfare. Animals 2022, 12, 3115. https://doi.org/10.3390/ani12223115
Mincu M, Gavojdian D, Nicolae I, Olteanu AC, Bota A, Vlagioiu C. Water Buffalo Responsiveness during Milking: Implications for Production Outputs, Reproduction Fitness, and Animal Welfare. Animals. 2022; 12(22):3115. https://doi.org/10.3390/ani12223115
Chicago/Turabian StyleMincu, Madalina, Dinu Gavojdian, Ioana Nicolae, Alexandru Corneliu Olteanu, Adrian Bota, and Constantin Vlagioiu. 2022. "Water Buffalo Responsiveness during Milking: Implications for Production Outputs, Reproduction Fitness, and Animal Welfare" Animals 12, no. 22: 3115. https://doi.org/10.3390/ani12223115
APA StyleMincu, M., Gavojdian, D., Nicolae, I., Olteanu, A. C., Bota, A., & Vlagioiu, C. (2022). Water Buffalo Responsiveness during Milking: Implications for Production Outputs, Reproduction Fitness, and Animal Welfare. Animals, 12(22), 3115. https://doi.org/10.3390/ani12223115