Effect of Feeding System on Muscle Fiber Composition, Antioxidant Capacity, and Nutritional and Organoleptic Traits of Goat Meat
Abstract
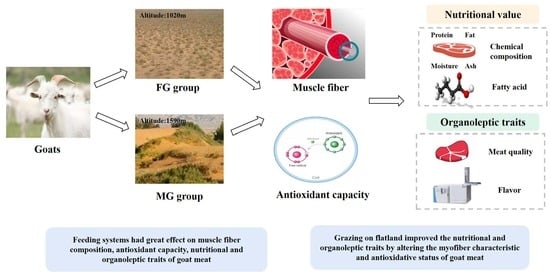
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Histochemical Analysis
2.4. Real-Time Quantitative PCR
2.5. Oxidative Stability Measurement
2.6. Determination of Meat Nutrition
2.7. Determination of Fatty Acids
2.8. Determination of Meat Quality
2.9. Determination of Volatile Flavor Compounds
2.10. Relative Odor Activity Value (ROAV) Analysis of Volatile Components
2.11. Statistical Analysis
3. Results
3.1. Muscle Fiber Characteristics
3.2. Oxidative Stability
3.3. Nutritional Traits
3.4. Fatty Acid Composition
3.5. Meat Quality
3.6. Volatile Flavor Compound
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Hou, C.; Ijaz, M.; Yan, T.; Li, X.; Li, Y.; Zhang, D. Proteomics discovery of protein biomarkers linked to meat quality traits in post-mortem muscles: Current trends and future prospects: A review. Trends Food Sci. Technol. 2020, 105, 416–432. [Google Scholar] [CrossRef]
- da Silva, L.O.; de Carvalho, G.G.P.; Tosto, M.S.L.; Lima, V.G.O.; Cirne, L.G.A.; de Araújo, M.L.G.M.L.; Pina, D.D.S.; Leite, V.M.; Rodrigues, C.S.; Mesquita, B.M.A.D.C. Effects of palm kernel cake in high-concentrate diets on carcass traits and meat quality of feedlot goats. Livest. Sci. 2021, 246, 104456. [Google Scholar] [CrossRef]
- Wang, B.; Wang., Z.; Chen., Y.; Liu, X.; Liu, K.; Zhang, Y.; Luo, H. Carcass traits, meat quality, and volatile compounds of lamb meat from different restricted grazing time and indoor supplementary feeding systems. Foods 2021, 10, 2822. [Google Scholar] [CrossRef]
- Simela, L.; Webb, E.C.; Frylinck, L. Effect of sex, age, and pre-slaughter conditioning on pH, temperature, tenderness and colour of indigenous South African goats. S. Afr. J. Anim. Sci. 2004, 34 (Suppl. 1), 208–211. [Google Scholar]
- Wang, B.; Yang, L.; Luo, Y.; Su, R.; Su, L.; Zhao, L.; Jin, Y. Effects of feeding regimens on meat quality, fatty acid composition and metabolism as related to gene expression in Chinese Sunit sheep. Small Rumin. Res. 2018, 169, 127–133. [Google Scholar] [CrossRef]
- Hou, Y.; Su, L.; Su, R.; Luo, Y.; Wang, B.; Yao, D.; Zhao, L.; Jin, Y. Effect of feeding regimen on meat quality, MyHC isoforms, AMPK, and PGC-1α genes expression in the biceps femoris muscle of Mongolia sheep. Food Sci. Nutr. 2020, 8, 2262–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.; Costa, A.F.; Lopes, P.A.; Alfaia, C.M.; Bessa, R.J.B.; Roseiro, L.C.; Prates, J.A.M. Fatty acid composition, cholesterol and α-tocopherol of Barrosã-PDO veal produced in farms located in lowlands, ridges and mountains. J. Food Compos. Anal. 2011, 24, 987–994. [Google Scholar] [CrossRef]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.-H.; Lee, K.-W.; Hwang, Y.-H.; Joo, S.-T. Histochemical Characteristics in Relation to Meat Quality Traits of Eight Major Muscles from Hanwoo Steers. Korean J. Food Sci. Anim. Resour. 2017, 37, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Dou, L.; Liu, C.; Yang, Z.; Su, R.; Chen, X.; Hou, Y.; Hu, G.; Yao, D.; Zhao, L.; Su, L.; et al. Effects of oxidative stability variation on lamb meat quality and flavour during post-mortem ageing. J. Food Sci. 2022, 87, 2578–2594. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, B.; Liu, C.; Su, R.; Hou, Y.; Yao, D.; Zhao, L.; Su, L.; Jin, Y. Meat quality, fatty acids, volatile compounds, and antioxidant properties of lambs fed pasture versus mixed diet. Food Sci. Nutr. 2019, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Luciano, G.; Vasta, V.; Monahan, F.J.; López-Andrés, P.; Biondi, L.; Lanza, M.; Priolo, A. Antioxidant status, colour stability and myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chem. 2011, 124, 1036–1042. [Google Scholar] [CrossRef]
- Zhao, H. Natural grassland resources and productivity in Urad Middle Banner, Inner Mongolia. Chin. J. Grassl. 1986, 1, 24–27. (In Chinese) [Google Scholar]
- Brooke, M.H.; Kaiser, K.K. Three “myosin adenosine triphosphatase” systems: The nature of their pH lability and sulfhydryl dependence. J. Histochem. Cytochem. 1970, 18, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G.; Dimauro, C.; Röhrle, F.; Priolo, A.; Monahan, F.J.; Moloney, A.P. The volatile profile of longissimus dorsi muscle of heifers fed pasture, pasture silage or cereal concentrate: Implication for dietary discrimination. Meat Sci. 2011, 87, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Zhou, G.H.; Xu, X.L. “ROAV” method: A new method for determining key odor compounds of rugao ham. Food Sci. 2008, 29, 370–374. (In Chinese) [Google Scholar]
- Gangnat, I.D.M.; Leiber, F.; Dufey, P.-A.; Silacci, P.; Kreuzer, M.; Berard, J. Physical activity, forced by steep pastures, affects muscle characteristics and meat quality of suckling beef calves. J. Agric. Sci. 2017, 155, 348–359. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, S.; Sun, Y.; Li, J.; Wu, D.; Shan, A.; Shi, B.; Cheng, B. Effects of dietary lecithin and L-camitine on fatty acid composition and lipid metabolic genes expression in subcutaneous fat and longissimus thoracis of growing-finishing pigs. Meat Sci. 2018, 136, 68–78. [Google Scholar] [CrossRef]
- Schmitt, A.; Herzog, P.; Röchner, F.; Brandle, A.-L.; Fragasso, A.; Munz, B. Skeletal muscle effects of two different 10-week exercise regimens, voluntary wheel running, and forced treadmill running, in mice: A pilot study. Physiol. Rep. 2020, 8, e14609. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Wen, W.; Luo, X.; Xia, B.; Guan, J.; Nie, Y.; Li, L.; Duan, J.; Suman, S.P.; Sun, Q. Post-mortem oxidative stability of three yak (Bos grunniens) muscles as influenced by animal age. Meat Sci. 2015, 105, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Muhlisin, D.T.U.; Lee, J.H.; Choi, J.H.; Utama, D.T.; Lee, S.K. Antioxidant enzyme activity, iron content and lipid oxidation of raw and cooked meat of Korean native chickens and other poultry. Asian-Austral. J. Anim. 2016, 29, 695–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, M.; Bashtani, M.; Naserian, A.A.; Farhangfar, H.; Emami, A. The effect of grapeseed oil on performance, rumen fermentation, antioxidant status and subcutaneous adipose fatty acid profile in lambs. J. Anim. Physiol. Anim. Nutr. 2017, 102, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.M.; Sancho, A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef]
- Nam, K.C.; Ahn, D.U. Effects of Ascorbic Acid and Antioxidants on the Color of Irradiated Ground Beef. J. Food Sci. 2003, 68, 1686–1690. [Google Scholar] [CrossRef]
- Liu, F.; Dai, R.; Zhu, J.; Li, X. Optimizing color and lipid stability of beef patties with a mixture design incorporating with tea catechins, carnosine, and α-tocopherol. J. Food Eng. 2010, 98, 170–177. [Google Scholar] [CrossRef]
- Kang, S.M.; Lee, I.S.; Lee, S.K. The quality characteristics of M. longissimus from Hanwoo (Korean cattle) steer with different raising altitudes and slaughter seasons. Livest. Sci. 2011, 136, 240–246. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Zhu, R.; Guo, J.; Wu, Y. Effect of myosin heavy chain composition of muscles on meat quality in Laiwu pigs and Duroc. Sci. China Life Sci. 2008, 51, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, F.; Ruano., J.; Perez-Martinez, P.; Lopez-Segura, F.; Lopez-Miranda, J. The influence of olive oil on human health:not a question of fat alone. Mol. Nutr. Food Res. 2007, 51, 1199–1208. [Google Scholar] [CrossRef]
- Cividini, A.; Levart, A.; Žgur, S.; Kompan, D. Fatty acid composition of lamb meat from the autochthonous Jezersko–Solčava breed reared in different production systems. Meat Sci. 2014, 97, 480–485. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris-Janzs, S.; Jones, P.J.H. Dietary monounsaturated fatty acids are protective against metabolic sy ndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef]
- Vahmani, P.; Ponnampalam, E.N.; Kraft, J.; Mapiye, C.; Bermingham, E.N.; Watkins, P.J.; Proctor, S.D.; Dugan, M.E.R. Bioactivity and Health Effects of Ruminant Meat Lipids. Invited Review. Meat Sci. 2020, 165, 108114. [Google Scholar] [CrossRef]
- Department of Health. Nutritional Aspects of Cardiovascular Disease. Report of Health and Social Subject No. 46; Her Majesty’s Stationery Office: London, UK, 1994. [Google Scholar]
- Ådnøy, T.; Haug, A.; Sørheim, O.; Thomassen, M.; Varszegi, Z.; Eik, L. Grazing on mountain pastures-does it affect meat quality in lambs? Livest. Prod. Sci. 2005, 94, 25–31. [Google Scholar] [CrossRef]
- Jaturasitha, S.; Norkeaw, R.; Vearasilp, T.; Wicke, M.; Kreuzer, M. Carcass and meat quality of Thai native cattle fattened on Guinea grass (Panicum maxima) or Guinea grass–legume (Stylosanthes guianensis) pastures. Meat Sci. 2009, 81, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Alasnier, C.; Rémignon, H.; Gandemer, G. Lipid characteristics associated with oxidative and glycolytic fibres in rabbit muscles. Meat Sci. 1996, 43, 213–224. [Google Scholar] [CrossRef]
- Leseigneur-Meynier, A.; Gandemer, G. Lipid composition of pork muscle in relation to the metabolic type of the fibres. Meat Sci. 1991, 29, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, Y.; Su, R.; Yao, D.; Hou, Y.; Liu, C.; Du, R.; Jin, Y. Impact of feeding regimens on the composition of gut microbiota and metabolite profiles of plasma and feces from Mongolian sheep. J. Microbiol. 2020, 58, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Rekiel, A.; Więcek, J.; Batorska, M.; Kulisiewicz, J. Effect of Piglet Birth Weight on Carcass Muscle and Fat Content and Pork Quality—A Review. Ann. Anim. Sci. 2015, 15, 271–287. [Google Scholar] [CrossRef] [Green Version]
- den Hertog-Meischke, M.J.A.; Smulders, F.J.M.; Houben, J.H.; Eikelenboom, G. The effect of dietary vitamin E supplementation on drip loss of bovine Longissimus lumborum, psoas major and Semitendinosus muscles. Meat Sci. 1997, 45, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kerth, C.R.; Wall, K.R.; Miller, R.K.; Whitney, T.R.; Stewart, W.C.; Boles, J.A.; Murphy, T.W. Effects of feeding juniper as a roughage on feedlot performance, carcass measurements, meat sensory attributes, and volatile aroma compounds of yearling Rambouillet wethers. J. Anim. Sci. 2019, 97, 2850–2864. [Google Scholar] [CrossRef] [Green Version]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Głąbska, D.; Brodowska, M.; Sun, D.-W.; Wierzbicka, A. Volatile compounds and fatty acids profile in Longissimus dorsi muscle from pigs fed with feed containing bioactive components. LWT 2016, 67, 112–117. [Google Scholar] [CrossRef]




| Group | Altitude (m) | Daily Mean Temperature (°C) | Mean Relative Humidity (%) | Mean Wind Speed (m/s) | Types of Forage Grass |
|---|---|---|---|---|---|
| FG | 1020 | 8.2 | 47.1 | 3.2 | Caragana interdia, Caragana stennophylla, Caragana brachypoda, Caragana intermedia, Caryopteris mongolica, Eurotia ceratoibes, Artemisiaordosica, Stipa gobica, Artemisia frigida, Cleistogenes mutica, Cleistogenes squarrosa, Ajania achilleoides, Agropyron desertorum, Aneurolepidium dasystachys |
| MG | 1590 | 2.0 | 58.6 | 5.4 | Caragana stennophylla, Stipa gobica, Convolvulus ammannii, Cleistogenes mutica, Cleistogenes squarrosa |
| Group | Crude Protein (%) | Crude Fat (%) | Carbohydrate (%) | High Digestible Fiber (%) | Low Digestible Fiber (%) | Lignin (%) | α-Tocopherol (mg/kg) | β-Carotene (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| FG | 12.7 | 3.5 | 19.1 | 10.7 | 50.2 | 7.9 | 13.2 | 450.8 |
| MG | 13.1 | 2.8 | 23.5 | 12.8 | 48.8 | 7.2 | 8.4 | 402.1 |
| Gene | Primer Sequence (5′-3′) | Product Length, bp | Genbank No. |
|---|---|---|---|
| GAPDH | F: CGGCACAGTCAAGGCAGAGAAC | 115 | XM_027961471.2 |
| R: CACGTACTCAGCACCAGCATCAC | |||
| MyHCI | F: CAACCTGGCTGATGCGGAAGAG | 111 | XM_004010325.4 |
| R: TTCATCTCCTCCTCGTCCTCCAAC | |||
| MyHCIIa | F: ACAGTACGAGGAGGAGCAGGAATC | 106 | XM_027974884.2 |
| R: GATGGCGTCCGTCTCATACTTGG | |||
| MyHCIIb | F: GACATCACGCAAATCCAGGGAGAG | 105 | XM_027974883.2 |
| R: CTCAGCCATCATAGCCGCATCAG | |||
| MyHCIIx | F: TTCCAGAAACCCAAACCTGCCAAG | 101 | XM_027974882.2 |
| R: TTGTCCAGCCAGCCAGTAATGTTG |
| Items | FG Group | MG Group | SEM | p-Value |
|---|---|---|---|---|
| Moisture (%) | 71.55 | 72.70 | 0.39 | 0.148 |
| Ash (%) | 1.16 | 1.05 | 0.03 | 0.188 |
| Protein (%) | 22.07 | 20.02 | 0.38 | 0.001 |
| Fat (%) | 5.72 | 4.19 | 0.39 | 0.068 |
| Fatty Acids (%) | FG Group | MG Group | SEM | p-Value |
|---|---|---|---|---|
| C14:0 | 2.11 | 1.79 | 0.07 | 0.012 |
| C15:0 | 0.32 | 0.28 | 0.03 | 0.577 |
| C16:0 | 19.17 | 27.92 | 1.06 | <0.001 |
| C17:0 | 1.07 | 0.90 | 0.04 | 0.017 |
| C18:0 | 16.40 | 16.03 | 0.41 | 0.677 |
| C24:0 | 0.66 | 0.93 | 0.08 | 0.156 |
| C16:1 | 1.37 | 1.44 | 0.06 | 0.629 |
| C17:1 | 0.71 | 0.85 | 0.04 | 0.099 |
| C18:1 n9t | 2.70 | 0.99 | 0.28 | <0.001 |
| C18:1 n9c | 46.14 | 41.89 | 1.12 | 0.044 |
| C20:1 | 1.03 | 1.37 | 0.08 | 0.015 |
| C18:2 n6t | 0.13 | ND | - | NS |
| C18:2 n6c | 5.51 | 2.71 | 0.19 | <0.001 |
| C18:3 n-3 | 0.60 | 0.62 | 0.03 | 0.834 |
| C20:3 n-3 | 2.08 | 2.28 | 0.15 | 0.610 |
| Ʃ SFA | 39.73 | 47.85 | 1.14 | 0.037 |
| Ʃ MUFA | 51.95 | 46.54 | 0.97 | 0.003 |
| Ʃ PUFA | 8.32 | 5.61 | 0.70 | 0.005 |
| n-6:n-3 | 2.10 | 0.93 | 0.26 | <0.001 |
| P:S | 0.21 | 0.12 | 0.02 | 0.027 |
| Items | FG Group | MG Group | SEM | p-Value |
|---|---|---|---|---|
| pH45min | 6.94 | 6.71 | 0.05 | 0.022 |
| pH24h | 5.65 | 5.66 | 0.05 | 0.961 |
| L* | 30.71 | 32.02 | 0.45 | 0.153 |
| a* | 17.86 | 16.53 | 0.23 | 0.002 |
| b* | 2.91 | 3.48 | 0.12 | 0.009 |
| Shear force (N) | 77.14 | 89.560 | 2.95 | 0.030 |
| Cooking loss (%) | 35.49 | 36.80 | 0.83 | 0.463 |
| Volatile Compound | Name | Relative Content (%) | SEM | p-Value | |
|---|---|---|---|---|---|
| FG Group | MG Group | ||||
| Aldehydes | Hexanal | 6.25 | 9.55 | 0.96 | 0.009 |
| Heptanal | 1.50 | 2.16 | 0.24 | 0.220 | |
| (E)-2-Octenal | 0.99 | 1.36 | 0.16 | 0.332 | |
| Octanal | 0.61 | 1.53 | 0.28 | 0.040 | |
| Nonanal | 9.87 | 10.06 | 0.11 | 0.491 | |
| Decanal | 5.15 | 5.82 | 0.39 | 0.510 | |
| (E)-2-Nonenal | 8.18 | 11.48 | 0.97 | 0.078 | |
| 10-Octadecenal | 0.86 | 0.71 | 0.08 | 0.407 | |
| Butanal-3-methyl | 1.50 | 5.65 | 1.21 | 0.023 | |
| Alcohols | 1-Pentanol | 2.83 | 4.41 | 0.39 | 0.033 |
| 1-Hexanol | 2.00 | 3.66 | 0.52 | 0.079 | |
| 1-Octen-3-ol | 16.48 | 13.67 | 0.71 | 0.006 | |
| 1-Octanol | 9.39 | 14.83 | 1.10 | 0.001 | |
| 1-Heptanol | 2.95 | 7.67 | 1.37 | 0.005 | |
| 2-Hexadecanol | 2.74 | 1.44 | 0.39 | 0.049 | |
| 1-Hexanol-2-ethyl | 1.61 | 5.03 | 1.00 | 0.013 | |
| 2,3-Butanediol | 2.55 | ND | - | NS | |
| 3-Octyn-2-ol | ND | 1.50 | - | NS | |
| Z,Z-2,5-Pentadecadien-1-ol | 2.10 | 0.64 | 0.44 | 0.051 | |
| Ketones | Acetone | 2.47 | 2.56 | 0.13 | NS |
| 2-Hexanone, 5-methy | 0.23 | ND | - | NS | |
| Acids | Pentadecanoic acid | 3.39 | 3.50 | 0.23 | 0.864 |
| Octadecanoic acid | 4.28 | ND | - | NS | |
| Esters | Carbamodithioic acid, diethyl-, methyl ester | 7.52 | 5.98 | 0.45 | 0.077 |
| Hexanoic acid, ethyl ester | 3.52 | 4.13 | 0.19 | 0.079 | |
| Phthalic acid, hexyl propyl ester | 3.96 | ND | - | NS | |
| Decanoic acid, octyl ester | 3.57 | ND | - | NS | |
| Others | Toluene | 1.15 | 1.50 | 0.11 | 0.047 |
| Ethylbenzene | 2.10 | 2.55 | 0.21 | 0.382 | |
| p-Xylene | 2.49 | 2.55 | 0.13 | 0.863 | |
| o-Xylene | 3.63 | 3.60 | 0.17 | 0.984 | |
| α-Muurolene | 2.44 | ND | - | NS | |
| Octadecane, 6-methyl | 0.58 | ND | - | NS | |
| Butane | 1.24 | ND | - | NS | |
| Ethanolamine | 1.56 | 1.75 | 0.11 | 0.484 | |
| Amphetamine | ND | 1.70 | - | NS | |
| Formamide, N,N-dibutyl | 8.95 | 9.20 | 0.24 | 0.678 | |
| Volatile Compound | Threshold Value (ng/g) | Odor Descriptors | ROAV | |
|---|---|---|---|---|
| FG Group | MG Group | |||
| Hexanal | 10 | Grassy | 3.793 | 3.627 |
| Heptanal | 3 | Jasmine, mint, burnt fat, green | 3.039 | 2.739 |
| (E)-2-Octenal | 3 | Wet ground, bitter, grass, meat, coffee | 2.011 | 1.726 |
| Octanal | 0.7 | Citrus-like, green, nutty, fatty | 5.280 | 8.325 |
| Nonanal | 1 | Rose fragrance | 59.914 | 38.226 |
| Decanal | 0.1 | Sweet Orange, Lemon, Rose, Wax | 70.219 | 69.098 |
| (E)-2-Nonenal | 0.08 | Fatty, tallow | 92.022 | 100 |
| 1-Pentanol | 4000 | Fuel oil, fruit, balsamic | 0.004 | 0.004 |
| 1-Hexanol | 500 | Woody, grass, fatty, fruity | 0.024 | 0.028 |
| 1-Octen-3-ol | 1 | Mushroom, smoke | 100 | 51.928 |
| 1-Octanol | 126 | Fatty, waxy, walnut, burnt | 0.452 | 0.435 |
| 1-Heptanol | 520 | Fragrant, woody, green, fatty | 0.034 | 0.055 |
| 1-Hexanol, 2-ethyl | 270,000 | Sweet, floral | <0.001 | <0.001 |
| Toluene | 1550 | Sweet | 0.004 | 0.004 |
| Ethylbenzene | 2205.25 | Sweet | 0.006 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, L.; Jin, Y.; Li, H.; Liu, C.; Yang, Z.; Chen, X.; Sun, L.; Zhao, L.; Su, L. Effect of Feeding System on Muscle Fiber Composition, Antioxidant Capacity, and Nutritional and Organoleptic Traits of Goat Meat. Animals 2023, 13, 172. https://doi.org/10.3390/ani13010172
Dou L, Jin Y, Li H, Liu C, Yang Z, Chen X, Sun L, Zhao L, Su L. Effect of Feeding System on Muscle Fiber Composition, Antioxidant Capacity, and Nutritional and Organoleptic Traits of Goat Meat. Animals. 2023; 13(1):172. https://doi.org/10.3390/ani13010172
Chicago/Turabian StyleDou, Lu, Ye Jin, Huijiao Li, Chang Liu, Zhihao Yang, Xiaoyu Chen, Lina Sun, Lihua Zhao, and Lin Su. 2023. "Effect of Feeding System on Muscle Fiber Composition, Antioxidant Capacity, and Nutritional and Organoleptic Traits of Goat Meat" Animals 13, no. 1: 172. https://doi.org/10.3390/ani13010172
APA StyleDou, L., Jin, Y., Li, H., Liu, C., Yang, Z., Chen, X., Sun, L., Zhao, L., & Su, L. (2023). Effect of Feeding System on Muscle Fiber Composition, Antioxidant Capacity, and Nutritional and Organoleptic Traits of Goat Meat. Animals, 13(1), 172. https://doi.org/10.3390/ani13010172