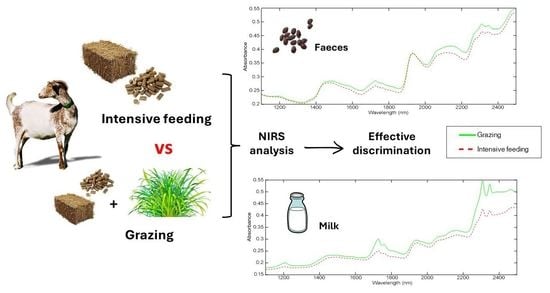
Preliminary Feasibility of Near-Infrared Spectroscopy to Authenticate Grazing in Dairy Goats through Milk and Faeces Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Sample Processing and Near-Infrared Spectra Collection
2.3. Data Processing and Discriminant Models Development
3. Results
3.1. NIRS Spectra Evaluation
3.2. PCA Using NIRS Spectra of Faeces and Milk Samples
3.3. Discriminant Models
3.3.1. Faeces Samples
3.3.2. Milk Samples
4. Discussion
4.1. NIRS Spectra Evaluation
4.2. PCA Using NIRS Spectra of Faeces and Milk Samples
4.3. Discriminant Models
4.3.1. Faeces Samples
4.3.2. Milk Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vlachos, A.; Arvanitoyannis, I.S.; Tserkezou, P. An Updated Review of Meat Authenticity Methods and Applications. Crit. Rev. Food Sci. Nutr. 2016, 56, 1061–1096. [Google Scholar] [CrossRef] [PubMed]
- Cardin, M.; Cardazzo, B.; Mounier, J.; Novelli, E.; Coton, M.; Coton, E. Authenticity and Typicity of Traditional Cheeses: A Review on Geographical Origin Authentication Methods. Foods 2022, 11, 3379. [Google Scholar] [CrossRef] [PubMed]
- Schiano, A.N.; Harwood, W.S.; Gerard, P.D.; Drake, M.A. Consumer Perception of the Sustainability of Dairy Products and Plant-Based Dairy Alternatives. J. Dairy Sci. 2020, 103, 11228–11243. [Google Scholar] [CrossRef]
- Yakubu, H.G.; Kovacs, Z.; Toth, T.; Bazar, G. The Recent Advances of Near-Infrared Spectroscopy in Dairy Production—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 810–831. [Google Scholar] [CrossRef]
- Rychlik, T.; Szwengiel, A.; Bednarek, M.; Arcuri, E.; Montet, D.; Mayo, B.; Nowak, J.; Czarnecki, Z. Application of the PCR-DGGE Technique to the Fungal Community of Traditional Wielkopolska Fried Ripened Curd Cheese to Determine Its PGI Authenticity. Food Control 2017, 73, 1074–1081. [Google Scholar] [CrossRef]
- Sreenivasan Tantuan, S.; Viljoen, C.D. Determining the Presence of Undeclared Animal Species Using Real-Time PCR in Canned and Ready-to-Eat Meat Products in South Africa. J. Food Sci. Technol. 2021, 58, 2699–2704. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Sentandreu, E. Authenticity of Meat Products: Tools against Fraud. Food Res. Int. 2014, 60, 19–29. [Google Scholar] [CrossRef]
- Coppa, M.; Martin, B.; Agabriel, C.; Chassaing, C.; Sibra, C.; Constant, I.; Graulet, B.; Andueza, D. Authentication of Cow Feeding and Geographic Origin on Milk Using Visible and Near-Infrared Spectroscopy. J. Dairy Sci. 2012, 95, 5544–5551. [Google Scholar] [CrossRef]
- Martin, B.; Fedele, V.; Ferlay, A.; Grolier, P.; Rock, E.; Gruffat, D.; Chilliard, Y. Effects of Grass-Based Diets on the Content of Micronutrients and Fatty Acids in Bovine and Caprine Dairy Products. In Land Use Systems in Grassland Dominated Regions, Proceedings of the 20th General Meeting of the European Grassland Federation, Luzern, Switzerland, 21–24 June 2004; Vdf Hochschulverlag AG an der ETH Zurich: Zurich, Switzerland, 2004; pp. 876–886. [Google Scholar]
- Andueza, D.; Agabriel, C.; Constant, I.; Lucas, A.; Martin, B. Using Visible or near Infrared Spectroscopy (NIRS) on Cheese to Authenticate Cow Feeding Regimes. Food Chem. 2013, 141, 209–214. [Google Scholar] [CrossRef]
- Elgersma, A.; Tamminga, S.; Ellen, G. Modifying Milk Composition through Forage. Anim. Feed Sci. Technol. 2006, 131, 207–225. [Google Scholar] [CrossRef]
- Vinci, G.; Preti, R.; Tieri, A.; Vieri, S. Authenticity and Quality of Animal Origin Food Investigated by Stable-Isotope Ratio Analysis. J. Sci. Food Agric. 2013, 93, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Cossignani, L.; Pollini, L.; Blasi, F. Invited Review: Authentication of Milk by Direct and Indirect Analysis of Triacylglycerol Molecular Species. J. Dairy Sci. 2019, 102, 5871–5882. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.T.; Moloney, A.P.; Brennan, L.; Monahan, F.J. Authentication of Beef Production Systems Using a Metabolomic-Based Approach. Animal 2012, 6, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Carpio, A.; Bonilla-Valverde, D.; Arce, C.; Rodríguez-Estévez, V.; Sánchez-Rodríguez, M.; Arce, L.; Valcárcel, M. Evaluation of Hippuric Acid Content in Goat Milk as a Marker of Feeding Regimen. J. Dairy Sci. 2013, 96, 5426–5434. [Google Scholar] [CrossRef]
- Capuano, E.; Van Der Veer, G.; Boerrigter-Eenling, R.; Elgersma, A.; Rademaker, J.; Sterian, A.; Van Ruth, S.M. Verification of Fresh Grass Feeding, Pasture Grazing and Organic Farming by Cows Farm Milk Fatty Acid Profile. Food Chem. 2014, 164, 234–241. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Brusic, V.; Georgiou, C.A. Food Authentication: State of the Art and Prospects. Curr. Opin. Food Sci. 2016, 10, 22–31. [Google Scholar] [CrossRef]
- Woodcock, T.; Downey, G.; O’Donnell, C.P. Better Quality Food and Beverages: The Role of near Infrared Spectroscopy. J. Near Infrared Spectrosc. 2008, 16, 1–29. [Google Scholar] [CrossRef]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2002; pp. 30–33. [Google Scholar]
- Pasquini, C. Near Infrared Spectroscopy: A Mature Analytical Technique with New Perspectives—A Review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Kademi, H.I.; Ulusoy, B.H.; Hecer, C. Applications of Miniaturized and Portable near Infrared Spectroscopy (NIRS) for Inspection and Control of Meat and Meat Products. Food Rev. Int. 2018, 35, 201–220. [Google Scholar] [CrossRef]
- Prieto, N.; Pawluczyk, O.; Dugan, M.E.R.; Aalhus, J.L. A Review of the Principles and Applications of Near-Infrared Spectroscopy to Characterize Meat, Fat, and Meat Products. Appl. Spectrosc. 2017, 71, 1403–1426. [Google Scholar] [CrossRef]
- Cozzolino, D.; Corbella, E.; Smyth, H.E. Quality Control of Honey Using Infrared Spectroscopy: A Review. Appl. Spectrosc. Rev. 2011, 46, 523–538. [Google Scholar] [CrossRef]
- Xu, H.R.; Wang, H.J.; Huang, K.; Ying, Y.B.; Yang, C.; Qian, H.; Hu, J. Comparison of PLS and SMLR for Nondestructive Determination of Sugar Content in Honey Peach Using NIRS. Guang Pu Xue Yu Guang Pu Fen Xi/Spectrosc. Spectr. Anal. 2008, 28, 2523–2526. [Google Scholar] [CrossRef]
- Prache, S.; Martin, B.; Coppa, M. Review: Authentication of Grass-Fed Meat and Dairy Products from Cattle and Sheep. Animal 2020, 14, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Schiborra, A.; Bulang, M.; Berk, A.; Susenbeth, A.; Schlecht, E. Using Faecal Near-Infrared Spectroscopy (FNIRS) to Estimate Nutrient Digestibility and Chemical Composition of Diets and Faeces of Growing Pigs. Anim. Feed Sci. Technol. 2015, 210, 234–242. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Martínez Marín, A.L.; Hernández, M.P.; Carrion, D.; Castro, G.G.; Pérez Alba, L.M. Faecal near Infrared Spectroscopy (NIRS) as a Tool to Asses Rabbit’s Feed Digestibility. Livest. Sci. 2012, 150, 386–390. [Google Scholar] [CrossRef]
- Dixon, R.; Coates, D. Review: Near Infrared Spectroscopy of Faeces to Evaluate the Nutrition and Physiology of Herbivores. J. Near Infrared Spectrosc. 2009, 17, 1–31. [Google Scholar]
- Núñez-Sánchez, N.; Carrion, D.; Peña Blanco, F.; Domenech García, V.; Garzón Sigler, A.; Martínez-Marín, A.L. Evaluation of Botanical and Chemical Composition of Sheep Diet by Using Faecal near Infrared Spectroscopy. Anim. Feed Sci. Technol. 2016, 222, 1–6. [Google Scholar] [CrossRef]
- Ottavian, M.; Franceschin, E.; Signorin, E.; Segato, S.; Berzaghi, P.; Contiero, B.; Cozzi, G. Application of near Infrared Reflectance Spectroscopy (NIRS) on Faecal Samples from Lactating Dairy Cows to Assess Two Levels of Concentrate Supplementation during Summer Grazing in Alpine Pastures. Anim. Feed Sci. Technol. 2015, 202, 100–105. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, P.; Cardador, M.J.; Arce, L.; Rodríguez-Estévez, V. Analytical Tools for Disease Diagnosis in Animals via Fecal Volatilome. Crit. Rev. Anal. Chem. 2020, 52, 917–932. [Google Scholar] [CrossRef]
- Rodríguez, M.S.; Genís, J.M.C. Comportamiento Alimentario Del Caprino y Alimentación En Sistemas de Pastoreo. Tierras Caprino 2013, 3, 34–45. [Google Scholar]
- Núñez-Sánchez, N.; Serradilla, J.M.; Ares, J.L.; Garrido-Varo, A. Effect of Moisture Uptake on the Repeatability of near Infrared Spectroscopy Analyses of Ewe Milk Using the Dry Extract System for Infrared (DESIR) Method. J. Near Infrared Spectrosc. 2008, 16, 381–388. [Google Scholar] [CrossRef]
- Williams, P.C. Implementation of Near-Infrared Technology. In Near Infrared Technology in the Agricultural and Food Industries; Williams, P., Norris, K.H., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 2001; pp. 145–169. [Google Scholar]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Westad, F.; Schmidt, A.; Kermit, M. Incorporating Chemical Band-Assignment in near Infrared Spectroscopy Regression Models. J. Near Infrared Spectrosc. 2008, 16, 265–273. [Google Scholar] [CrossRef]
- Berzaghi, P.; Dalle Zotte, A.; Jansson, L.M.; Andrighetto, I. Near-Infrared Reflectance Spectroscopy as a Method to Predict Chemical Composition of Breast Meat and Discriminate between Different n-3 Feeding Sources. Poult. Sci. 2005, 84, 128–136. [Google Scholar] [CrossRef]
- Decruyenaere, V.; Lecomte, P.; Demarquilly, C.; Aufrere, J.; Dardenne, P.; Stilmant, D.; Buldgen, A. Evaluation of Green Forage Intake and Digestibility in Ruminants Using near Infrared Reflectance Spectroscopy (NIRS): Developing a Global Calibration. Anim. Feed Sci. Technol. 2009, 148, 138–156. [Google Scholar] [CrossRef]
- Coppa, M.; Chassaing, C.; Sibra, C.; Cornu, A.; Verbič, J.; Golecký, J.; Engel, E.; Ratel, J.; Boudon, A.; Ferlay, A.; et al. Forage System Is the Key Driver of Mountain Milk Specificity. J. Dairy Sci. 2019, 102, 10483–10499. [Google Scholar] [CrossRef] [PubMed]
- Peinado-Lucena, E.; Sánchez-Rodríguez, M.; Gómez-Castro, A.G.; Mata-Moreno, C.; Gallego-Barrera, J.A. Dry Matter Intake per Mouthful by Grazing Dairy Goats. Small Rumin. Res. 1992, 7, 215–223. [Google Scholar] [CrossRef]
- Peinado Lucena, E.; Sánchez Rodríguez, M.; Gómez-Castro, A.G.; Alcalde Leal, J.L.; Mata Moreno, C. Evolución Estacional Del Pastoreo y Producción de Un Rebaño Caprino Lechero En Áreas Adehesadas. Arch. Zootec. 1990, 39, 25–34. [Google Scholar]
- Sánchez Rodríguez, M.; Gómez-Castro, A.G.; Peinado Lucena, E.; Domenech García, V.; Mata Moreno, C. Evolución de La Selección de Plantas Leñosas Por Caprinos de Aptitud Lechera En Pastoreo. Arch. Zootec. 1992, 41, 607–618. [Google Scholar]
- Mata Moreno, C.; Gómez-Castro, A.G.; Peinado Lucena, E.; Sánchez Rodríguez, M.; Domenech García, V.; Martínez Teruel, M. Análisis de La Evolución Del Comportamiento Selectivo de Un Rebaño de Ganado Caprino a Lo Largo de La Jornada de Pastoreo. Arch. Zootec. 1991, 40, 273–281. [Google Scholar]
- Celaya, R.; Benavides, R.; García, U.; Ferreira, L.M.M.; Ferre, I.; Martínez, A.; Ortega-Mora, L.M.; Osoro, K. Grazing Behaviour and Performance of Lactating Suckler Cows, Ewes and Goats on Partially Improved Heathlands. Animal 2008, 2, 1818–1831. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, P.; Saavedra, D.; Martín-Gómez, A.; Cardador, M.J.; Arce, L.; Rodríguez-Estévez, V. In Vivo Authentication of Iberian Pig Feeding Regime Using Faecal Volatilome Information. Livest. Sci. 2022, 260, 104913. [Google Scholar] [CrossRef]
- Landau, S.; Glasser, T.; Dvash, L. Monitoring Nutrition in Small Ruminants with the Aid of near Infrared Reflectance Spectroscopy (NIRS) Technology: A Review. Small Rumin. Res. 2006, 61, 1–11. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Cipolat-Gotet, C.; Cecchinato, A.; Schiavon, S.; Bittante, G. Chemometric Authentication of Farming Systems of Origin of Food (Milk and Ripened Cheese) Using Infrared Spectra, Fatty Acid Profiles, Flavor Fingerprints, and Sensory Descriptions. Food Chem. 2020, 305, 125480. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, A.; Delgadillo-Puga, C.; Decandia, M.; Molle, G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals 2019, 9, 771. [Google Scholar] [CrossRef]
- Engel, E.; Ferlay, A.; Cornu, A.; Chilliard, Y.; Agabriel, C.; Bielicki, G.; Martin, B. Relevance of Isotopic and Molecular Biomarkers for the Authentication of Milk According to Production Zone and Type of Feeding of the Cow. J. Agric. Food Chem. 2007, 55, 9099–9108. [Google Scholar] [CrossRef]
- Segato, S.; Galaverna, G.; Contiero, B.; Berzaghi, P.; Caligiani, A.; Marseglia, A.; Cozzi, G. Identification of Lipid Biomarkers to Discriminate between the Different Production Systems for Asiago PDO Cheese. J. Agric. Food Chem. 2017, 65, 9887–9892. [Google Scholar] [CrossRef]
- Coppa, M.; Chassaing, C.; Ferlay, A.; Agabriel, C.; Laurent, C.; Borreani, G.; Barcarolo, R.; Baars, T.; Kusche, D.; Harstad, O.M.; et al. Potential of Milk Fatty Acid Composition to Predict Diet Composition and Authenticate Feeding Systems and Altitude Origin of European Bulk Milk. J. Dairy Sci. 2015, 98, 1539–1551. [Google Scholar] [CrossRef]
- Coppa, M.; Ferlay, A.; Borreani, G.; Revello-Chion, A.; Tabacco, E.; Tornambé, G.; Pradel, P.; Martin, B. Effect of Phenological Stage and Proportion of Fresh Herbage in Cow Diets on Milk Fatty Acid Composition. Anim. Feed Sci. Technol. 2015, 208, 66–78. [Google Scholar] [CrossRef]



| Algorithm | Mathematical Treatment | Classification Success | |||
|---|---|---|---|---|---|
| Faeces Samples | Milk Samples | ||||
| Model Calibration | Model External Validation | Model Calibration | Model External Validation | ||
| PLS2 | 1, 5, 1, 1 no scatter | 100% | 81.8% | 78.6% | 100% |
| 2, 5, 1, 1 no scatter | 100% | 100% | 94.6% | 100% | |
| 1, 5, 1, 1 SNV + DT | 100% | 100% | 98.2% | 73.3% | |
| 2, 5, 1, 1 SNV + DT | 96.4% | 90.9% | 80.4% | 73.3% | |
| Correlation | 1, 5, 1, 1 no scatter | 87.3% | 90.9% | 87.5% | 93.3% |
| 2, 5, 1, 1 no scatter | 90.9% | 81.8% | 92.9% | 100% | |
| 1, 5, 1, 1 SNV + DT | 87.3% | 90.9% | 85.7% | 93.3% | |
| 2, 5, 1, 1 SNV + DT | 90.9% | 81.8% | 87.5% | 100% | |
| Maximum distance | 1, 5, 1, 1 no scatter | 94.5% | 81.8% | 94.6% | 100% |
| 2, 5, 1, 1 no scatter | 96.4% | 81.8% | 78.6% | 80% | |
| 1, 5, 1, 1 SNV + DT | 96.4% | 72.7% | 98.2% | 100% | |
| 2, 5, 1, 1 SNV + DT | 94.5% | 54.5% | 94.6% | 80% | |
| Mahalanobis distance | 1, 5, 1, 1 no scatter | 89.1% | 90.9% | 87.5% | 80% |
| 2, 5, 1, 1 no scatter | 89.1% | 90.9% | 76.8% | 73.3% | |
| 1, 5, 1, 1 SNV + DT | 87.3% | 100% | 92.9% | 93.3% | |
| 2, 5, 1, 1 SNV + DT | 87.3% | 54.5% | 85.7% | 73.3% | |
| X-residuals | 1, 5, 1, 1 no scatter | 100% | 90.9% | 92.9% | 73.3% |
| 2, 5, 1, 1 no scatter | 100% | 100% | 89.3% | 100% | |
| 1, 5, 1, 1 SNV + DT | 100% | 100% | 98.2% | 100% | |
| 2, 5, 1, 1 SNV + DT | 100% | 100% | 98.2% | 93.3% | |
| Maximum X-residuals | 1, 5, 1, 1 no scatter | 96.4% | 90.9% | 100% | 86.7% |
| 2, 5, 1, 1 no scatter | 98.2% | 90.9% | 96.4% | 93.3% | |
| 1, 5, 1, 1 SNV + DT | 96.4% | 100% | 100% | 93.3% | |
| 2, 5, 1, 1 SNV + DT | 100% | 100% | 98.2% | 80% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Hernández, P.; Díaz-Gaona, C.; Reyes-Palomo, C.; Sanz-Fernández, S.; Sánchez-Rodríguez, M.; Rodríguez-Estévez, V.; Núñez-Sánchez, N. Preliminary Feasibility of Near-Infrared Spectroscopy to Authenticate Grazing in Dairy Goats through Milk and Faeces Analysis. Animals 2023, 13, 2440. https://doi.org/10.3390/ani13152440
Rodríguez-Hernández P, Díaz-Gaona C, Reyes-Palomo C, Sanz-Fernández S, Sánchez-Rodríguez M, Rodríguez-Estévez V, Núñez-Sánchez N. Preliminary Feasibility of Near-Infrared Spectroscopy to Authenticate Grazing in Dairy Goats through Milk and Faeces Analysis. Animals. 2023; 13(15):2440. https://doi.org/10.3390/ani13152440
Chicago/Turabian StyleRodríguez-Hernández, Pablo, Cipriano Díaz-Gaona, Carolina Reyes-Palomo, Santos Sanz-Fernández, Manuel Sánchez-Rodríguez, Vicente Rodríguez-Estévez, and Nieves Núñez-Sánchez. 2023. "Preliminary Feasibility of Near-Infrared Spectroscopy to Authenticate Grazing in Dairy Goats through Milk and Faeces Analysis" Animals 13, no. 15: 2440. https://doi.org/10.3390/ani13152440
APA StyleRodríguez-Hernández, P., Díaz-Gaona, C., Reyes-Palomo, C., Sanz-Fernández, S., Sánchez-Rodríguez, M., Rodríguez-Estévez, V., & Núñez-Sánchez, N. (2023). Preliminary Feasibility of Near-Infrared Spectroscopy to Authenticate Grazing in Dairy Goats through Milk and Faeces Analysis. Animals, 13(15), 2440. https://doi.org/10.3390/ani13152440