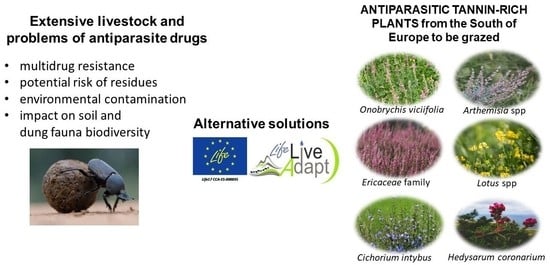
Antiparasitic Tannin-Rich Plants from the South of Europe for Grazing Livestock: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Plants Containing Secondary Metabolites (PSM) in the Mediterranean Region
2.1. Condensed Tannins (CT)
2.1.1. Factors Involved in the Antiparasitic Effect of Condensed Tannins
2.1.2. Factors Affecting the Condensed Tannins Content
2.1.3. Other Effects of Condensed Tannins
2.1.4. Other Factors Affecting Tannin Action
3. Antiparasitic Mechanism of Plants Rich in Secondary Compounds
4. Anti-Parasite Plants in Mediterranean Region
4.1. Arthemisia spp.
| In Vivo/In Vitro | Animal Species | Indoor/Outdoor | Natural Infection/Experimental Infection | Parasites | Specific Parasite | Dosage | References |
|---|---|---|---|---|---|---|---|
| In vitro | - | - | - | Protozoa | Leishmania infantum, Trypanosoma cruzi | 800, 400, 100 μg/mL | [140] |
| L. infantum, Trypanosoma cruzi | 800, 400, and 100 μg/mL | [141] | |||||
| Leishmania aethiopica and Leishmania donovani | 0.0097–0.1565 μL/mL and EC50 0.24–42.00 nl/mL | [146] | |||||
| Goat | - | - | GIN | Haemonchus contortus | 0.5, 1, 2, and 4 mg/mL | [81] | |
| Sheep | - | - | GIN | H. contortus | 0.34% DM | [126] | |
| 25 mg/mL and 50 mg/mL | [148] | ||||||
| CAE 25 mg/mL and CME 25 mg/mL | [149] | ||||||
| CAE 25 mg/mL and CME 25 mg/mL | [150] | ||||||
| In vivo | - | - | Natural infection | GIN | - | 50 mL/P.O. of A. campestris macerate as single dose | [151] |
| Cattle | Outdoor | Natural infection | GIN | - | 100–150 mg/kg per subject twice in two weeks | [11] | |
| Gerbil | - | - | GIN | H. contortus | 160 mg/mL | [143] | |
| Goat | Outdoor | Natural infection | GIN | H. contortus and Teladorsagia circumcincta | 4 mg/kg of n-hexane extract of A. cina (Acn-h) | [81] | |
| Mice | Indoor | - | GIN | Hymenolepis nana, Aspiculuris tetraptera, Syphacia obvelata | 150 mg/kg | [152] | |
| Rat | - | - | GIN | Trichinella spiralis | 300 and 600 mg/kg | [147] | |
| Sheep | - | - | GIN | H. contortus, Trichostrongylus colubriformis, Trichostrongylus axei, Oesophagostomum columbianum, Strongyloides papillosus and Trichuris ovis | CAE and CME 2.0 and 3.0 g | [149] | |
| H. contortus, T. ovis, Chabertia ovina, Bunostomum trigonocephalum y O. columbianum | CAE and CEE at 1.0 and 2.0 g kg−1 BW | [150] | |||||
| Platelmint | Fasciola hepatica | 160 mg/kg (intramuscular dose) | [131] | ||||
| Natural infection | GIN | - | 4, 6 and 8% DM | [148] | |||
| Indoor | Experimental infection | GIN | H. contortus | 0.34% DM | [126] |
4.2. Cichorium intybus L.
4.3. Ericaceae Family
4.4. Hedysarum coronarium L.
| In Vivo/In Vitro | Animal Species | Indoor/Outdoor | Natural Infection/Experimental Infection | Parasites | Specific Parasite | Dosage | References |
|---|---|---|---|---|---|---|---|
| In vitro | Deer | - | - | Lung worms | Dictyocaulus stages viviparus | 1200 μg/mL | [78] |
| Sheep | - | - | GIN | Haemonchus contortus, Ostertagia circumcincta and Trichostrongylus colubriformis | 50, 100, 200, 400, 800 and 1000 mg CT/mL | [56] | |
| T. colubriformis | 200, 400 μg/mL | [72] | |||||
| H. contortus | 3.3% DM | [126] | |||||
| O. circumcincta; T. colubriformis | 3.13, 3.51% DM | [133] | |||||
| T. colubriformis | 400, 800, 1000 μg/mL | [186] | |||||
| In vivo | Deer | Indoor | Experimental infection | GIN | - | 3.49% DM | [117] |
| Lung worms | Dictyocaulus sp | 3.49% DM | [117] | ||||
| Goat | Indoor | Natural infection | GIN | Teladorsagia circumcincta, T.colubriformis, T. vitrinus and Trichuris spp. | 26 g/kg free CT, 18 g/kg protein-bound CT and 1 g/kg fibre-bound CT | [185] | |
| Sheep | Indoor | Experimental infection | GIN | H. contortus | 3.3% DM | [126] | |
| O. circumcincta; T. colubriformis | 3.13, 3.51% DM | [133] | |||||
| T. colubriformis | 3% DM | [135] | |||||
| O. circumcincta and T. colubriformis | - | [187] | |||||
| Outdoor | Experimental infection | GIN | T. axei, O. circumcincta and T. colubriformis (experimento 2) | 9.93 and 12.05% DM | [4] | ||
| T. colubriformis | 15.8% DM | [125] | |||||
| O. circumcincta; T. colubriformis | 3.13, 3.51% DM | [133] | |||||
| T. circumcincta | 36.9 g/kg DM | [167] | |||||
| T. circumcincta | 15.8 g/kg DM | [168] | |||||
| T. colubriformis and O. circumcincta | - | [188] | |||||
| Natural infection | GIN | T. axei, O. circumcincta and T. colubriformis (experimento 2) | 9.93 and 12.05% DM | [4] | |||
| Ostertagia, Haemonchus, Trichostrongylus, Nematodirus, Cooperia | - | [137] | |||||
| T. colubriformis and O. circumcincta | - | [188] | |||||
| - | - | [189] |
4.5. Lotus spp.
4.6. Onobrychis viciifolia Scop.
5. Future Research and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arroyo-Lopez, C.; Manolaraki, F.; Saratsis, A.; Saratsi, K.; Stefanakis, A.; Skampardonis, V.; Voutzourakis, N.; Hoste, H.; Sotiraki, S. Anthelmintic Effect of Carob Pods and Sainfoin Hay When Fed to Lambs after Experimental Trickle Infections with Haemonchus Contortus and Trichostrongylus Colubriformis. Parasite 2014, 21, 71. [Google Scholar] [CrossRef] [Green Version]
- Desrues, O.; Peña-Espinoza, M.; Hansen, T.V.A.; Enemark, H.L.; Thamsborg, S.M. Anti-Parasitic Activity of Pelleted Sainfoin (Onobrychis Viciifolia) against Ostertagia Ostertagi and Cooperia Oncophora in Calves. Parasites Vectors 2016, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Supali, T.; Verweij, J.J.; Wiria, A.E.; Djuardi, Y.; Hamid, F.; Kaisar, M.M.M.; Wammes, L.J.; van Lieshout, L.; Luty, A.J.F.; Sartono, E.; et al. Polyparasitism and Its Impact on the Immune System. Int. J. Parasitol. 2010, 40, 1171–1176. [Google Scholar] [CrossRef]
- Niezen, J.H.; Waghorn, T.S.; Charleston, W.A.G.; Waghorn, G.C. Growth and Gastrointestinal Nematode Parasitism in Lambs Grazing Either Lucerne (Medicago Sativa) or Sulla (Hedysarum Coronarium) Which Contains Condensed Tannins. J. Agric. Sci. 1995, 125, 281–289. [Google Scholar] [CrossRef]
- Kimambo, A.E.; MacRae, J.C.; Walker, A.; Watt, C.F.; Coop, R.L. Effect of Prolonged Subclinical Infection with Trichostrongylus Colubriformis on the Performance and Nitrogen Metabolism of Growing Lambs. Vet. Parasitol. 1988, 28, 191–203. [Google Scholar] [CrossRef]
- MacRae, J.C. Metabolic Consequences of Intestinal Parasitism. Proc. Nutr. Soc. 1993, 52, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Coop, R.L.; Kyriazakis, I. Influence of Host Nutrition on the Development and Consequences of Nematode Parasitism in Ruminants. Trends Parasitol. 2001, 17, 325–330. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.; Paolini, V.; Aguilar-Caballero, A.; Etter, E.; Lefrileux, Y.; Chartier, C.; Broqua, C. Interactions between Nutrition and Gastrointestinal Infections with Parasitic Nematodes in Goats. Small Rumin. Res. 2005, 60, 141–151. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial Assessment of the Economic Burden of Major Parasitic Helminth Infections to the Ruminant Livestock Industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Paolini, V.; Bergeaud, J.P.; Grisez, C.; Prevot, F.; Dorchies, P.; Hoste, H. Effects of Condensed Tannins on Goats Experimentally Infected with Haemonchus Contortus. J. Vet. Parasitol. 2003, 113, 253–261. [Google Scholar] [CrossRef]
- Anagonou, S.I.N.; Adenile, D.A.; Daga, D.F.; Odoumbourou, M.S.; Akouedegni, C.G.; Hounzangbe-Adote, S. Comparative Study of the Effects of Albendazole and Annual Mug Wort (Artemisia Annua) Powder on Gastrointestinal Nematodes in Cattle. World J. Biol. Pharm. Health Sci. 2022, 10, 61–66. [Google Scholar] [CrossRef]
- Hovi, M.; Sundrum, A.; Thamsborg, S.M. Animal Health and Welfare in Organic Livestock Production in Europe: Current State and Future Challenges. Livest. Prod. Sci. 2003, 80, 41–53. [Google Scholar] [CrossRef] [Green Version]
- European Parlament; European Council Regulation (EU). 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007; European Parlament: Strasbourg, France, 2018; pp. 1–92. [Google Scholar]
- Hughes, P.L.; Dowling, A.F.; Callinan, A.P.L. Resistance to Macrocyclic Lactone Anthelmintics and Associated Risk Factors on Sheep Farms in the Lower North Island of New Zealand. New Zealand Vet. J. 2007, 55, 177–183. [Google Scholar] [CrossRef]
- Jackson, F.; Varady, M.; Bartley, D.J. Managing Anthelmintic Resistance in Goats—Can We Learn Lessons from Sheep? Small Rumin. Res. 2012, 103, 3–9. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Vidyashankar, A.N. An Inconvenient Truth: Global Worming and Anthelmintic Resistance. Vet. Parasitol. 2012, 186, 70–78. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Thamsborg, S.M.; Demeler, J.; Enemark, H.L. Field Efficacy of Four Anthelmintics and Confirmation of Drug-Resistant Nematodes by Controlled Efficacy Test and Pyrosequencing on a Sheep and Goat Farm in Denmark. Vet. Parasitol. 2014, 206, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Gaudin, E.; Simon, M.; Quijada, J.; Schelcher, F.; Sutra, J.F.; Lespine, A.; Hoste, H. Efficacy of Sainfoin (Onobrychis Viciifolia) Pellets against Multi Resistant Haemonchus Contortus and Interaction with Oral Ivermectin: Implications for on-Farm Control. Vet. Parasitol. 2016, 227, 122–129. [Google Scholar] [CrossRef]
- Waller, P.J. From Discovery to Development: Current Industry Perspectives for the Development of Novel Methods of Helminth Control in Livestock. Vet. Parasitol. 2006, 139, 1–14. [Google Scholar] [CrossRef]
- Kaminsky, R.; Ducray, P.; Jung, M.; Clover, R.; Rufener, L.; Bouvier, J.; Weber, S.S.; Wenger, A.; Wieland-Berghausen, S.; Goebel, T.; et al. A New Class of Anthelmintics Effective against Drug-Resistant Nematodes. Nature 2008, 452, 176–180. [Google Scholar] [CrossRef]
- Scott, I.; Pomroy, W.E.; Kenyon, P.R.; Smith, G.; Adlington, B.; Moss, A. Lack of Efficacy of Monepantel against Teladorsagia Circumcincta and Trichostrongylus Colubriformis. Vet. Parasitol. 2013, 198, 166–171. [Google Scholar] [CrossRef]
- Van den Brom, R.; Moll, L.; Kappert, C.; Vellema, P. Haemonchus Contortus Resistance to Monepantel in Sheep. Vet. Parasitol. 2015, 209, 278–280. [Google Scholar] [CrossRef]
- Martínez-Valladares, M.; Geurden, T.; Bartram, D.J.; Martínez-Pérez, J.M.; Robles-Pérez, D.; Bohórquez, A.; Florez, E.; Meana, A.; Rojo-Vázquez, F.A. Resistance of Gastrointestinal Nematodes to the Most Commonly Used Anthelmintics in Sheep, Cattle and Horses in Spain. Vet. Parasitol. 2015, 211, 228–233. [Google Scholar] [CrossRef]
- Stampa, E.; Schipmann-Schwarze, C.; Hamm, U. Consumer Perceptions, Preferences, and Behavior Regarding Pasture-Raised Livestock Products: A Review. Food Qual. Prefer. 2020, 82, 103872. [Google Scholar] [CrossRef]
- Waller, P.J.; Thamsborg, S.M. Nematode Control in ‘Green’ Ruminant Production Systems. Trends Parasitol. 2004, 20, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The Effects of Tannin-Rich Plants on Parasitic Nematodes in Ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- McKellar, Q.A. Ecotoxicology and Residues of Anthelmintic Compounds. Vet. Parasitol. 1997, 72, 413–435. [Google Scholar] [CrossRef]
- Lumaret, J.P.; Errouissi, F. Use of Anthelmintics in Herbivores and Evaluation of Risks for the Non Target Fauna of Pastures. Vet. Res. 2002, 33, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin Residues Disrupt Dung Beetle Diversity, Soil Properties and Ecosystem Functioning: An Interdisciplinary Field Study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Sanz-Fernández, S.; Reyes-Palomo, C.; Díaz-Gaona, C.; Rodríguez Estévez, V. Antiparasitarios Alternativos. Fertil. De La Tierra Rev. De Agric. Ecológica 2020, 81, 20–23. [Google Scholar]
- Sanz-Fernández, S.; Reyes-Palomo, C.; Díaz-Gaona, C.; Rodríguez-Estévez, V. Plantas Antiparasitarias Para La Ganadería Extensiva. AE Rev. Agroecol. Divulg. 2019, 38, 47. [Google Scholar]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant Bioactives for Ruminant Health and Productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadou, S.; Gray, D.; Younie, D.; Tzamaloukas, O.; Jackson, F.; Kyriazakis, I. The Use of Chicory for Parasite Control in Organic Ewes and Their Lambs. Parasitology 2007, 134, 299–307. [Google Scholar] [CrossRef]
- Jayawardene, K.D.; Palombo, E.A.; Boag, P.R. Natural Products Are a Promising Source for Anthelmintic Drug Discovery. Biomolecules 2021, 11, 1457. [Google Scholar] [CrossRef]
- Terrill, T.H.; Dykes, G.S.; Shaik, S.A.; Miller, J.E.; Kouakou, B.; Kannan, G.; Burke, J.M.; Mosjidis, J.A. Efficacy of Sericea Lespedeza Hay as a Natural Dewormer in Goats: Dose Titration Study. Vet. Parasitol. 2009, 163, 52–56. [Google Scholar] [CrossRef]
- Terrill, T.H.; Mosjidis, J.A.; Moore, D.A.; Shaik, S.A.; Miller, J.E.; Burke, J.M.; Muir, J.P.; Wolfe, R. Effect of Pelleting on Efficacy of Sericea Lespedeza Hay as a Natural Dewormer in Goats. Vet. Parasitol. 2007, 146, 117–122. [Google Scholar] [CrossRef]
- Brunet, S.; Jackson, F.; Hoste, H. Effects of Sainfoin (Onobrychis Viciifolia) Extract and Monomers of Condensed Tannins on the Association of Abomasal Nematode Larvae with Fundic Explants. Int. J. Parasitol. 2008, 38, 783–790. [Google Scholar] [CrossRef]
- Brunet, S.; De Montellano, C.M.O.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Aguilar-Caballero, A.J.; Capetillo-Leal, C.; Hoste, H. Effect of the Consumption of Lysiloma Latisiliquum on the Larval Establishment of Gastrointestinal Nematodes in Goats. Vet. Parasitol. 2008, 157, 81–88. [Google Scholar] [CrossRef]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An Appraisal of Natural Products Active against Parasitic Nematodes of Animals. Parasites Vectors 2019, 12, 306. [Google Scholar] [CrossRef] [Green Version]
- Githiori, J.B.; Athanasiadou, S.; Thamsborg, S.M. Use of Plants in Novel Approaches for Control of Gastrointestinal Helminths in Livestock with Emphasis on Small Ruminants. Vet. Parasitol. 2006, 139, 308–320. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Concentration, and Diet Composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef] [Green Version]
- Oliveira Santos, F.; Ponce Morais Cerqueira, A.; Branco, A.; José Moreira Batatinha, M.; Borges Botura, M. Anthelmintic Activity of Plants against Gastrointestinal Nematodes of Goats: A Review. Parasitology 2019, 146, 1233–1246. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Antiparasitic Effects of Medicinal Plants (Part 1)-A Review. J. Pharm. 2016, 6, 51–66. [Google Scholar]
- Manolaraki, F.; Sotiraki, S.; Stefanakis, A.; Skampardonis, V.; Volanis, M.; Hoste, H. Anthelmintic Activity of Some Mediterranean Browse Plants against Parasitic Nematodes. Parasitology 2010, 137, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.J.; Miller, J.; Ungar, E.D.; Landau, S.Y.; Glendinning, J. Ruminant Self-Medication against Gastrointestinal Nematodes: Evidence, Mechanism, and Origins. Parasite 2014, 21, 31. [Google Scholar] [CrossRef] [Green Version]
- Torres-Fajardo, R.A.; González-Pech, P.G.; Sandoval-Castro, C.A.; Ventura-Cordero, J.; Torres-Acosta, J.F.J. Criollo Goats Limit Their Grass Intake in the Early Morning Suggesting a Prophylactic Self-Medication Behaviour in a Heterogeneous Vegetation. Trop. Anim. Health Prod. 2019, 51, 2473–2479. [Google Scholar] [CrossRef]
- Moreno-Gonzalo, J.; Ferre, I.; Celaya, R.; Frutos, P.; Ferreira, L.M.M.; Hervás, G.; García, U.; Ortega-Mora, L.M.; Osoro, K. Potential Use of Heather to Control Gastrointestinal Nematodes in Goats. Small Rumin. Res. 2012, 103, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.; Vogl, C.R.; Amorena, M.; Hamburger, M.; Walkenhorst, M. Treatment of Organic Livestock with Medicinal Plants: A Systematic Review of European Ethnoveterinary Research. Forsch. Komplementmed. 2014, 21, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhang, D.; Cai, X.; Xia, L.; Luo, Y.; Cheng, X.; An, S. Significant Alterations in Soil Fungal Communities along a Chronosequence of Spartina Alterniflora Invasion in a Chinese Yellow Sea Coastal Wetland. Sci. Total Environ. 2019, 693, 133548. [Google Scholar] [CrossRef]
- Acamovic, T.; Brooker, J.D. Biochemistry of Plant Secondary Metabolites and Their Effects in Animals. Proc. Nutr. Soc. 2005, 64, 403–412. [Google Scholar] [CrossRef]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of Rumen Fermentation and Methane Production with Plant Secondary Metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Barrau, E.; Fabre, N.; Fouraste, I.; Hoste, H. Effect of Bioactive Compounds from Sainfoin (Onobrychis Viciifolia Scop.) on the in Vitro Larval Migration of Haemonchus Contortus: Role of Tannins and Flavonol Glycosides. Parasitology 2005, 131, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Brunet, S.; Hoste, H. Monomers of Condensed Tannins Affect the Larval Exsheathment of Parasitic Nematodes of Ruminants. J. Agric. Food Chem. 2006, 54, 7481–7487. [Google Scholar] [CrossRef]
- Moreno-Gonzalo, J.; Manolaraki, F.; Frutos, P.; Hervás, G.; Celaya, R.; Osoro, K.; Ortega-Mora, L.M.; Hoste, H.; Ferre, I. In Vitro Effect of Heather (Ericaceae) Extracts on Different Development Stages of Teladorsagia Circumcincta and Haemonchus Contortus. Vet. Parasitol. 2013, 197, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Meagher, L.P.; Spencer, P.A.; Sivakumaran, S. Effect of Flavan-3-Ols on in Vitro Egg Hatching, Larval Development and Viability of Infective Larvae of Trichostrongylus Colubriformis. Int. J. Parasitol. 2003, 33, 1691–1698. [Google Scholar] [CrossRef]
- Molan, A.L.; Alexander, R.A.; Brookes, I.M.; McNabb, W.C. Effects of an Extract from Sulla (Hedysarum Coronarium) Containing Condensed Tannins on the Migration of Three Sheep Gastrointestinal Nematodes in Vitro. Proc. New Zealand Soc. Anim. Prod. 2000, 60, 21–25. [Google Scholar]
- Rufino-Moya, P.J. Influencia Del Tipo de Forraje y de La Presencia de Taninos Condensados En La Fermentación in Vitro de Las Dietas de Ovino En El Área Mediterránea. Uso de Biomarcadores Para La Trazabilidad Del Sistema de Alimentación. Doctoral dissertation, Universidad de Zaragoza, Zaragoza, Spain, 2019. [Google Scholar]
- Mueller-Harvey, I.; McAllan, A.B. Tannins: Their Biochemistry and Nutritional Properties. Adv. Plant Cell Biochem. Biotechnol. 1992, 1, 151–217. [Google Scholar]
- Desrues, O.; Fryganas, C.; Ropiak, H.M.; Mueller-Harvey, I.; Enemark, H.L.; Thamsborg, S.M. Impact of Chemical Structure of Flavanol Monomers and Condensed Tannins on in Vitro Anthelmintic Activity against Bovine Nematodes. Parasitology 2016, 143, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckendorn, F.; Häring, D.A.; Maurer, V.; Senn, M.; Hertzberg, H. Individual Administration of Three Tanniferous Forage Plants to Lambs Artificially Infected with Haemonchus Contortus and Cooperia Curticei. Vet. Parasitol. 2007, 146, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Saratsis, A.; Regos, I.; Tzanidakis, N.; Voutzourakis, N.; Stefanakis, A.; Treuter, D.; Joachim, A.; Sotiraki, S. In Vivo and in Vitro Efficacy of Sainfoin (Onobrychis Viciifolia) against Eimeria Spp in Lambs. Vet. Parasitol. 2012, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Markovics, A.; Cohen, I.; Muklada, H.; Glasser, T.A.; Dvash, L.; Ungar, E.D.; Azaizeh, H.; Landau, S.Y. Consumption of Pistacia Lentiscus Foliage Alleviates Coccidiosis in Young Goats. Vet. Parasitol. 2012, 186, 165–169. [Google Scholar] [CrossRef]
- Burke, J.; Miller, J.E.; Terrill, T.H.; Orlik, S.T.; Acharya, M.; Garza, J.J.; Mosjidis, J.A. Sericea Lespdeza as an Aid in the Control of Emeria Spp. in Lambs. Vet. Parasitol. 2013, 193, 39–46. [Google Scholar] [CrossRef]
- Kommuru, D.S.; Barker, T.; Desai, S.; Burke, J.M.; Ramsay, A.; Mueller-Harvey, I.; Miller, J.E.; Mosjidis, J.A.; Kamisetti, N.; Terrill, T.H. Use of Pelleted Sericea Lespedeza (Lespedeza Cuneata) for Natural Control of Coccidia and Gastrointestinal Nematodes in Weaned Goats. Vet. Parasitol. 2014, 204, 191–198. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P. Tannins for Suppression of Internal Parasites. J. Anim. Sci. 2003, 81, E102–E109. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional Aspects of Ecologically Relevant Phytochemicals in Ruminant Production. Front. Vet. Sci. 2021, 8, 155. [Google Scholar] [CrossRef]
- Gaudin, E.; Costes-Thiré, M.; Villalba, J.J.; Hoste, H.; Gerfault, V.; Ginane, C. Relative Abilities of Young Sheep and Goats to Self-Medicate with Tannin-Rich Sainfoin When Infected with Gastrointestinal Nematodes. Animal 2019, 13, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalo, J.; Osoro, K.; García, U.; Frutos, P.; Celaya, R.; Ferreira, L.M.M.; Ortega-Mora, L.M.; Ferre, I. Effect of the Consumption of Heather on Incoming Larvae and Established Population of Teladorsagia Circumcincta in Experimentally Infected Cashmere Goats. Vet. Parasitol. 2013, 196, 124–129. [Google Scholar] [CrossRef]
- Paolini, V.; Prevot, F.; Dorchies, P.; Hoste, H. Lack of Effects of Quebracho and Sainfoin Hay on Incoming Third-Stage Larvae of Haemonchus Contortus in Goats. Vet. J. 2005, 170, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Paolini, V.; Dorchies, P.; Hoste, H.; Paolini, V.; Dorchies, P.; Hoste, H. Effects of sainfoin hay on gastrointestinal infection with nematodes in goats. Vet. Rec. 2003, 152, 600–601. [Google Scholar] [CrossRef]
- Kidane, A.; Houdijk, J.G.M.; Athanasiadou, S.; Tolkamp, B.J.; Kyriazakis, I. Effects of Maternal Protein Nutrition and Subsequent Grazing on Chicory (Cichorium Intybus) on Parasitism and Performance of Lambs. J. Anim. Sci. 2010, 88, 1513–1521. [Google Scholar] [CrossRef]
- Molan, A.L.; Waghorn, G.C.; McNabb, W.C. Effect of Condensed Tannins on Egg Hatching and Larval Development of Trichostrongylus Colubriformis in Vitro. Vet. Rec. 2002, 150, 65–69. [Google Scholar] [CrossRef]
- Bahuaud, D.; Martinez-Ortiz De Montellano, C.; Chauveau, S.; Prevot, F.; Torres-Acosta, F.; Fouraste, I.; Hoste, H. Effects of Four Tanniferous Plant Extracts on the in Vitro Exsheathment of Third-Stage Larvae of Parasitic Nematodes. Parasitology 2006, 132, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Novobilský, A.; Mueller-Harvey, I.; Thamsborg, S.M. Condensed Tannins Act against Cattle Nematodes. Vet. Parasitol. 2011, 182, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Capetillo-Leal, C.; Brunet, S.; Hoste, H. Effects of Four Tropical Tanniniferous Plant Extracts on the Inhibition of Larval Migration and the Exsheathment Process of Trichostrongylus Colubriformis Infective Stage. Vet. Parasitol. 2008, 153, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Barone, C.D.; Zajac, A.M.; Ferguson, S.M.; Brown, R.N.; Reed, J.D.; Krueger, C.G.; Petersson, K.H. In Vitro Screening of 51 Birdsfoot Trefoil (Lotus Corniculatus L.; Fabaceae) Strains for Anti-Parasitic Effects against Haemonchus Contortus. Parasitology 2019, 146, 828–836. [Google Scholar] [CrossRef]
- Brunet, S.; Aufrere, J.; El Babili, F.; Fouraste, I.; Hoste, H. The Kinetics of Exsheathment of Infective Nematode Larvae Is Disturbed in the Presence of a Tannin-Rich Plant Extract (Sainfoin) Both in Vitro and in Vivo. Parasitology 2007, 134, 1253–1262. [Google Scholar] [CrossRef]
- Molan, A.L.; Hoskin, S.O.; Barry, T.N.; McNabb, W.C. Effect of Condensed Tannins Extracted from Four Forages on the Viability of the Larvae of Deer Lungworms and Gastrointestinal Nematodes. Vet. Rec. 2000, 147, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Paolini, V.; Fouraste, I.; Koste, H. In Vitro Effects of Three Woody Plant and Sainfoin Extracts on 3rd-Stage Larvae and Adult Worms of Three Gastrointestinal Nematodes. Parasitology 2004, 129, 69–77. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.; Alonso-diaz, M.Á.; Brunet, S.; Sandoval-Castro, C.; Adote, S.H. Identification and Validation of Bioactive Plants for the Control of Gastrointestinal Nematodes in Small Ruminants. Trop. Biomed. 2008, 25, 56–72. [Google Scholar]
- Higuera-Piedrahita, R.I.; Dolores-Hernández, M.; de la-Cruz-Cruz, H.A.; Andrade-Montemayor, H.M.; Zamilpa, A.; López-Arellano, R.; González-Garduño, R.; Cuéllar-Ordaz, J.A.; Mendoza-de-Gives, P.; López-Arellano, M.E. An Artemisia Cina N-Hexane Extract Reduces the Haemonchus Contortus and Teladorsagia Circumcincta Fecal Egg Count in Naturally Infected Periparturient Goats. Trop. Anim. Health Prod. 2022, 54, 95. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Boas, U.; Williams, A.R.; Thamsborg, S.M.; Simonsen, H.T.; Enemark, H.L. Sesquiterpene Lactone Containing Extracts from Two Cultivars of Forage Chicory (Cichorium Intybus) Show Distinctive Chemical Profiles and in Vitro Activity against Ostertagia Ostertagi. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Lonngren, K.J.; Barone, C.D.; Zajac, A.M.; Brown, R.N.; Reed, J.D.; Krueger, C.G.; Petersson, K.H. Effect of Birdsfoot Trefoil Cultivars on Exsheathment of Haemonchus Contortus in Fistulated Sheep. Vet. Parasitol. 2020, 287, 109271. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Effects and Fate of Tannins in Ruminant Animals, Adaptation to Tannins, and Strategies to Overcome Detrimental Effects of Feeding Tannin-Rich Feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Kyriazakis, I. Plant Secondary Metabolites: Antiparasitic Effects and Their Role in Ruminant Production Systems. Proc. Nutr. Soc. 2004, 63, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertzberg, H.; Huwyler, U.; Kohler, L.; Rehbein, S.; Wanner, M. Kinetics of Exsheathment of Infective Ovine and Bovine Strongylid Larvae in Vivo and in Vitro. Parasitology 2002, 125, 65–70. [Google Scholar] [CrossRef]
- Hansen, T.V.A.; Fryganas, C.; Acevedo, N.; Caraballo, L.; Thamsborg, S.M.; Mueller-Harvey, I.; Williams, A.R. Proanthocyanidins Inhibit Ascaris Suum Glutathione-S-Transferase Activity and Increase Susceptibility of Larvae to Levamisole in Vitro. Parasitol. Int. 2016, 65, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Kabasa, J.D.; Opuda-Asibo, J.; Ter Meulen, U. The Effect of Oral Administration of Polyethylene Glycol on Faecal Helminth Egg Counts in Pregnant Goats Grazed on Browse Containing Condensed Tannins. Trop. Anim. Health Prod. 2000, 32, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Moya, P.J.; Blanco, M.; Bertolín, J.R.; Joy, M. Methane Production of Fresh Sainfoin, with or without PEG, and Fresh Alfalfa at Different Stages of Maturity Is Similar but the Fermentation End Products Vary. Animals 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazandarani, M.; Majidi, Z.; Zarghami-Moghaddam, P.; Abrodi, M.; Hemati, H.; Fathiazad, F. Essential Oil Composition, Total Phenol, Flavonoid, Anthocyanin and Antioxidant Activities in Different Parts of Artemisia Annua L. in Two Localities (North of Iran). J. Med. Plants By-Prod. 2012, 1, 13–21. [Google Scholar] [CrossRef]
- Salah, L.; Eddine, L.S.; Redha, O.M.; Ladjel, S. Influence of Solvent Extraction on Phenolic Content, Antioxidant and Anti-Inflammatory Activities of Aerial Parts Extract from Algerian Artemisia Herba-Alba. J. Pharm. Res. 2016, 10, 58–64. [Google Scholar]
- Sunmonu, T.O.; Afolayan, A.J. Evaluation of Polyphenolic Content and Antioxidant Activity of Artemisia Afra Jacq. Ex Willd. Aqueous Extract. Pak. J. Nutr. 2012, 11, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Theodoridou, K.; Aufrère, J.; Niderkorn, V.; Andueza, D.; Le Morvan, A.; Picard, F.; Baumont, R. In Vitro Study of the Effects of Condensed Tannins in Sainfoin on the Digestive Process in the Rumen at Two Vegetation Cycles. Anim. Feed Sci. Technol. 2011, 170, 147–159. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Ramos, G.; Giráldez, F.J.; Mantecón, A.R. Condensed Tannin Content of Several Shrub Species from a Mountain Area in Northern Spain, and Its Relationship to Various Indicators of Nutritive Value. Anim. Feed Sci. Technol. 2002, 95, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Rufino-Moya, P.J.; Blanco, M.; Bertolín, J.R.; Joy, M. Effect of the Method of Preservation on the Chemical Composition and in Vitro Fermentation Characteristics in Two Legumes Rich in Condensed Tannins. Anim. Feed Sci. Technol. 2019, 251, 12–20. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. An in Vitro Study on the Ability of Polyethylene Glycol to Inhibit the Effect of Quebracho Tannins and Tannic Acid on Rumen Fermentation in Sheep, Goats, Cows, and Deer. Aust. J. Agric. Res. 2004, 55, 1125–1132. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia Absinthium L.) Essential Oils and Phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar] [CrossRef] [Green Version]
- Jonker, A.; Yu, P. The Occurrence, Biosynthesis, and Molecular Structure of Proanthocyanidins and Their Effects on Legume Forage Protein Precipitation, Digestion and Absorption in the Ruminant Digestive Tract. Int. J. Mol. Sci. 2017, 18, 1105. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, S.S.; Sathe, S.K.; Salunkhe, D.K. Chemistry and Safety of Plant Polyphenols. Adv. Exp. Med. Biol. 1984, 177, 457–495. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The Effect of Condensed Tannins on the Nutrition and Health of Ruminants Fed Fresh Temperate Forages: A Review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Waghorn, G.C.; McNabb, W.C. Consequences of Plant Phenolic Compounds for Productivity and Health of Ruminants. Proc. Nutr. Soc. 2003, 62, 383–392. [Google Scholar] [CrossRef]
- Ketzis, J.K.; Vercruysse, J.; Stromberg, B.E.; Larsen, M.; Athanasiadou, S.; Houdijk, J.G.M. Evaluation of Efficacy Expectations for Novel and Non-Chemical Helminth Control Strategies in Ruminants. Vet. Parasitol. 2006, 139, 321–335. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the Conundrum of Tannins in Animal Nutrition and Health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Barry, T.N.; McNabb, W.C. The Implications of Condensed Tannins on the Nutritive Value of Temperate Forages Fed to Ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Frutos, P.; Moreno-Gonzalo, J.; Hervás, G.; García, U.; Ferreira, L.M.M.; Celaya, R.; Toral, P.G.; Ortega-Mora, L.M.; Ferre, I.; Osoro, K. Is the Anthelmintic Effect of Heather Supplementation to Grazing Goats Always Accompanied by Anti-Nutritional Effects? Animal 2008, 2, 1449–1456. [Google Scholar] [CrossRef] [Green Version]
- Houdijk, J.G.M.; Athanasiadou, S. Direct and Indirect Effects of Host Nutrition on Ruminant Gastrointestinal Nematodes. In Proceedings of the VI International Symposium on the Nutrition of Herbivores, Mérida, México, October 2003; Universidad Autónoma de Yucatán: Mérida, México, 2003; pp. 213–236. [Google Scholar]
- Nawab, A.; Tang, S.; Gao, W.; Li, G.; Xiao, M.; An, L.; Wu, J.; Liu, W. Tannin Supplementation in Animal Feeding; Mitigation Strategies to Overcome the Toxic Effects of Tannins on Animal Health: A Review. J. Agric. Sci. 2020, 12, p217. [Google Scholar] [CrossRef] [Green Version]
- Lees, G.L. Condensed Tannins in Some Forage Legumes: Their Role in the Prevention of Ruminant Pasture Bloat. Basic Life Sci. 1992, 59, 915–934. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S. Methane Mitigation from Ruminants Using Tannins and Saponins. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-Analysis of the Relationship between Dietary Tannin Level and Methane Formation in Ruminants from in Vivo and in Vitro Experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Direct Anthelmintic Effects of Condensed Tannins towards Different Gastrointestinal Nematodes of Sheep: In Vitro and in Vivo Studies. Vet. Parasitol. 2001, 99, 205–219. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Effects of Short-Term Exposure to Condensed Tannins on Adult Trichostrongylus Colubriformis. Vet. Rec. 2000, 146, 728–732. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Consequences of Long-Term Feeding with Condensed Tannins on Sheep Parasitised with Trichostrongylus Colubriformis. Int. J. Parasitol. 2000, 30, 1025–1033. [Google Scholar] [CrossRef]
- Paolini, V.; Frayssines, A.; De La Farge, F.; Dorchies, P.; Hoste, H. Effects of Condensed Tannins on Established Populations and on Incoming Larvae of Trichostrongylus Colubriformis and Teladorsagia Circumcincta in Goats. Vet. Res. 2003, 34, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Hoskin, S.O.; Wilson, P.R.; Barry, T.N.; Charleston, W.A.G.; Waghorn, G.C. Effect of Forage Legumes Containing Condensed Tannins on Lungworm (Dictyocaulus Sp.) and Gastrointestinal Parasitism in Young Red Deer (Cervus Elaphus). Res. Vet. Sci. 2000, 68, 223–230. [Google Scholar] [CrossRef]
- Moreno-Gonzalo, J.; Manolaraki, F.; Frutos, P.; Hervás, G.; Celaya, R.; Osoro, K.; Ortega-Mora, L.M.; Hoste, H.; Ferre, I. In Vitro Effect of Heather Extracts on Trichostrongylus Colubriformis Eggs, Larvae and Adults. Vet. Parasitol. 2013, 197, 586–594. [Google Scholar] [CrossRef]
- Molan, A.L.; Sivakumaran, S.; Spencer, P.A.; Meagher, L.P. Green Tea Flavan-3-Ols and Oligomeric Proanthocyanidins Inhibit the Motility of Infective Larvae of Teladorsagia Circumcincta and Trichostrongylus Colubriformis in Vitro. Res. Vet. Sci. 2004, 77, 239–243. [Google Scholar] [CrossRef]
- Koupai-Abyazani, M.R.; Muir, A.D.; Bohm, B.A.; Towers, G.H.N.; Gruber, M.Y. The Proanthocyanidin Polymers in Some Species of Onobrychis. Phytochemistry 1993, 34, 113–117. [Google Scholar] [CrossRef]
- Marais, J.P.J.; Mueller-Harvey, I.; Brandt, E.V.; Ferreira, D. Polyphenols, Condensed Tannins, and Other Natural Products in Onobrychis Viciifolia (Sainfoin). J. Agric. Food Chem. 2000, 48, 3440–3447. [Google Scholar] [CrossRef]
- Gujja, S.; Terrill, T.H.; Mosjidis, J.A.; Miller, J.E.; Mechineni, A.; Kommuru, D.S.; Shaik, S.A.; Lambert, B.D.; Cherry, N.M.; Burke, J.M. Effect of Supplemental Sericea Lespedeza Leaf Meal Pellets on Gastrointestinal Nematode Infection in Grazing Goats. Vet. Parasitol. 2013, 191, 51–58. [Google Scholar] [CrossRef]
- Heckendorn, F.; Häring, D.A.; Maurer, V.; Zinsstag, J.; Langhans, W.; Hertzberg, H. Effect of Sainfoin (Onobrychis Viciifolia) Silage and Hay on Established Populations of Haemonchus Contortus and Cooperia Curticei in Lambs. Vet. Parasitol. 2006, 142, 293–300. [Google Scholar] [CrossRef]
- Häring, D.A.; Suter, D.; Amrhein, N.; Lüscher, A. Biomass Allocation Is an Important Determinant of the Tannin Concentration in Growing Plants. Ann. Bot. 2007, 99, 111–120. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Tzamaloukas, O.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Testing for Direct Anthelmintic Effects of Bioactive Forages against Trichostrongylus Colubriformis in Grazing Sheep. Vet. Parasitol. 2005, 127, 233–243. [Google Scholar] [CrossRef]
- Valderrábano, J.; Calvete, C.; Uriarte, J. Effect of Feeding Bioactive Forages on Infection and Subsequent Development of Haemonchus Contortus in Lamb Faeces. Vet. Parasitol. 2010, 172, 89–94. [Google Scholar] [CrossRef]
- Fitch, G.; Figueroa, L.L.; Koch, H.; Stevenson, P.C.; Adler, L.S. Understanding Effects of Floral Products on Bee Parasites: Mechanisms, Synergism, and Ecological Complexity. Int. J. Parasitol. Parasites Wildl. 2022, 17, 244–256. [Google Scholar] [CrossRef]
- Houdijk, J.G.M.; Jessop, N.S.; Kyriazakis, I.; Houdijk, J.G.M. Nutrient Partitioning between Reproductive and Immune Functions in Animals. Proc. Nutr. Soc. 2001, 60, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Van Houtert, M.F.J.; Barger, I.A.; Steel, J.W. Dietary Protein for Young Grazing Sheep: Interactions with Gastrointestinal Parasitism. Vet. Parasitol. 1995, 60, 283–295. [Google Scholar] [CrossRef]
- Valente, A.H.; de Roode, M.; Ernst, M.; Peña-Espinoza, M.; Bornancin, L.; Bonde, C.S.; Martínez-Valladares, M.; Ramünke, S.; Krücken, J.; Simonsen, H.T.; et al. Identification of Compounds Responsible for the Anthelmintic Effects of Chicory (Cichorium Intybus) by Molecular Networking and Bio-Guided Fractionation. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 105–114. [Google Scholar] [CrossRef]
- Keiser, J.; Rinaldi, L.; Veneziano, V.; Mezzino, L.; Tanner, M.; Utzinger, J.; Cringoli, G. Efficacy and Safety of Artemether against a Natural Fasciola Hepatica Infection in Sheep. Parasitol. Res. 2008, 103, 517–522. [Google Scholar] [CrossRef]
- Osoro, K.; Mateos-Sanz, A.; Frutos, P.; García, U.; Ortega-Mora, L.M.; Ferreira, L.M.M.; Celaya, R.; Ferre, I. Anthelmintic and Nutritional Effects of Heather Supplementation on Cashmere Goats Grazing Perennial Ryegrass-White Clover Pastures. J. Anim. Sci. 2007, 85, 861–870. [Google Scholar] [CrossRef]
- Niezen, J.H.; Charleston, W.A.G.; Robertson, H.A.; Shelton, D.; Waghorn, G.C.; Green, R. The Effect of Feeding Sulla (Hedysarum Coronarium) or Lucerne (Medicago Sativa) on Lamb Parasite Burdens and Development of Immunity to Gastrointestinal Nematodes. Vet. Parasitol. 2002, 105, 229–245. [Google Scholar] [CrossRef]
- Hoskin, S.O.; Barry, T.N.; Wilson, P.R.; Charleston, W.A.G.; Hodgson, J. Effects of Reducing Anthelmintic Input upon Growth and Faecal Egg and Larval Counts in Young Farmed Deer Grazing Chicory (Cichorium Intybus) and Perennial Ryegrass (Lolium Perenne)/White Clover (Trifolium Repens) Pasture. J. Agric. Sci. 1999, 132, 335–345. [Google Scholar] [CrossRef]
- Niezen, J.H.; Waghorn, G.C.; Graham, T.; Carter, J.L.; Leathwick, D.M. The Effect of Diet Fed to Lambs on Subsequent Development of Trichostrongylus Colubriformis Larvae in Vitro and on Pasture. Vet. Parasitol. 2002, 105, 269–283. [Google Scholar] [CrossRef] [PubMed]
- López, C.; Ferreira, L.M.M.; García, U.; Moreno-Gonzalo, J.; Rodrigues, M.A.M.; Osoro, K.; Ferre, I.; Celaya, R. Diet Selection and Performance of Horses Grazing on Different Heathland Types. Animal 2017, 11, 1708–1717. [Google Scholar] [CrossRef]
- Niezen, J.H.; Robertson, H.A.; Waghorn, G.C.; Charleston, W.A.G. Production, Faecal Egg Counts and Worm Burdens of Ewe Lambs Which Grazed Six Contrasting Forages. Vet. Parasitol. 1998, 80, 15–27. [Google Scholar] [CrossRef]
- Paolini, V.; De La Farge, F.; Prevot, F.; Dorchies, P.; Hoste, H. Effects of the Repeated Distribution of Sainfoin Hay on the Resistance and the Resilience of Goats Naturally Infected with Gastrointestinal Nematodes. Vet. Parasitol. 2005, 127, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Hoste, H.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Mueller-Harvey, I.; Sotiraki, S.; Louvandini, H.; Thamsborg, S.M.; Terrill, T.H. Tannin Containing Legumes as a Model for Nutraceuticals against Digestive Parasites in Livestock. Vet. Parasitol. 2015, 212, 5–17. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Bailen, M.; Diaz, C.E.; Fraga, B.M.; Martínez-Díaz, R.; Zuñiga, G.E.; Contreras, R.A.; Cabrera, R.; Burillo, J. Major Components of Spanish Cultivated Artemisia Absinthium Populations: Antifeedant, Antiparasitic, and Antioxidant Effects. Ind. Crops Prod. 2012, 37, 401–407. [Google Scholar] [CrossRef]
- Bailen, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martínez-Díaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical Composition and Biological Effects of Essential Oils from Artemisia Absinthium L. Cultivated under Different Environmental Conditions. Ind. Crops Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Agelet, A. Estudis d’etnobotànica Farmacèutica Al Pallars. Rev. D’etnologia De Catalunya 2000, 17, 120–123. [Google Scholar]
- Squires, J.M.; Ferreira, J.F.S.; Lindsay, D.S.; Zajac, A.M. Effects of Artemisinin and Artemisia Extracts on Haemonchus Contortus in Gerbils (Meriones Unguiculatus). Vet. Parasitol. 2011, 175, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lutgen, P. Tannins in Artemisia: The Hidden Treasure of Prophylaxis. Pharm. Pharmacol. Int. J. 2018, 6, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Boufennara, S.; Lopez, S.; Bousseboua, H.; Bodas Rodríguez, R.; Bouazza, L. Chemical Composition and Digestibility of Some Browse Plant Species Collected from Algerian Arid Rangelands. Span. J. Agric. Res. 2012, 10, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. In Vitro Evaluation of Antileishmanial Activity and Toxicity of Essential Oils of Artemisia Absinthium and Echinops Kebericho. Chem. Biodivers. 2011, 8, 614–623. [Google Scholar] [CrossRef]
- Caner, A.; Döşkaya, M.; Deǧirmenci, A.; Can, H.; Baykan, Ş.; Üner, A.; Başdemir, G.; Zeybek, U.; Gürüz, Y. Comparison of the Effects of Artemisia Vulgaris and Artemisia Absinthium Growing in Western Anatolia against Trichinellosis (Trichinella Spiralis) in Rats. Exp. Parasitol. 2008, 119, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.I.; Osman, S.A.; Al-Gaabary; El-Soud, M.H.; Abo, K.M. Effects of Chicory (Cichorium Intybus) and Artemisia Absenthium Extracts Against Ovine Gastrointestinal Nematodes. Int. J. Food Agric. Vet. Sci. 2014, 4, 43–53. [Google Scholar]
- Iqbal, Z.; Lateef, M.; Ashraf, M.; Jabbar, A. Anthelmintic Activity of Artemisia Brevifolia in Sheep. J. Ethnopharmacol. 2004, 93, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.A.; Chishti, M.Z.; Ahmad, F.; Shawl, A.S. Anthelmintic Activity of Extracts of Artemisia Absinthium against Ovine Nematodes. Vet. Parasitol. 2009, 160, 83–88. [Google Scholar] [CrossRef]
- Castagna, F.; Piras, C.; Palma, E.; Musolino, V.; Lupia, C.; Bosco, A.; Rinaldi, L.; Cringoli, G.; Musella, V.; Britti, D. Green Veterinary Pharmacology Applied to Parasite Control: Evaluation of Punica Granatum, Artemisia Campestris, Salix Caprea Aqueous Macerates against Gastrointestinal Nematodes of Sheep. Vet. Sci. 2021, 8, 237. [Google Scholar] [CrossRef]
- Amirmohammadi, M.; Khajoenia, S.; Bahmani, M.; Rafieian-Kopaei, M.; Eftekhari, Z.; Qorbani, M. In Vivo Evaluation of Antiparasitic Effects of Artemisia Abrotanum and Salvia Officinalis Extracts on Syphacia Obvelata, Aspiculoris Tetrapetra and Hymenolepis Nana Parasites. Asian Pac. J. Trop. Dis. 2014, 4, S250–S254. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L.; López-Muñoz, R.; Williams, A.R. Antiparasitic Activity of Chicory (Cichorium Intybus) and Its Natural Bioactive Compounds in Livestock: A Review. Parasites Vectors 2018, 11, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Kemp, P.D. Forage Chicory (Cichorium Intybus L.): A Review of Its Agronomy and Animal Production. Adv. Agron. 2005, 88, 187–222. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium Intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadou, S.; Githiori, J.; Kyriazakis, I. Medicinal Plants for Helminth Parasite Control: Facts and Fiction. Animal 2007, 1, 1392–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, S.B.; Harborne, J.B. The Role of Sesquiterpene Lactones and Phenolics in the Chemical Defence of the Chicory Plant. Phytochemistry 1985, 24, 2225–2231. [Google Scholar] [CrossRef]
- Scales, G.H.; Knight, T.L.; Saville, D.J. Effect of Herbage Species and Feeding Level on Internal Parasites and Production Performance of Grazing Lambs. New Zealand J. Agric. Res. 1995, 38, 237–247. [Google Scholar] [CrossRef]
- Marley, C.L.; Cook, R.; Barrett, J.; Keatinge, R.; Lampkin, N.H.; McBride, S.D. The Effect of Dietary Forage on the Development and Survival of Helminth Parasites in Ovine Faeces. Vet. Parasitol. 2003, 118, 93–107. [Google Scholar] [CrossRef]
- Marley, C.L.; Cook, R.; Keatinge, R.; Barrett, J.; Lampkin, N.H. The Effect of Birdsfoot Trefoil (Lotus Corniculatus) and Chicory (Cichorium Intybus) on Parasite Intensities and Performance of Lambs Naturally Infected with Helminth Parasites. Vet. Parasitol. 2003, 112, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Peña-Espinoza, M.; Valente, A.H.; Bornancin, L.; Simonsen, H.T.; Thamsborg, S.M.; Williams, A.R.; López-Muñoz, R. Anthelmintic and Metabolomic Analyses of Chicory (Cichorium Intybus) Identify an Industrial by-Product with Potent in Vitro Antinematodal Activity. Vet. Parasitol. 2020, 280, 109088. [Google Scholar] [CrossRef]
- Woolsey, I.D.; Valente, A.H.; Williams, A.R.; Thamsborg, S.M.; Simonsen, H.T.; Enemark, H.L. Anti-Protozoal Activity of Extracts from Chicory (Cichorium Intybus) against Cryptosporidium Parvum in Cell Culture. Sci. Rep. 2019, 9, 20414. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Williams, A.R.; Thamsborg, S.M.; Simonsen, H.T.; Enemark, H.L. Anthelmintic Effects of Forage Chicory (Cichorium Intybus) against Free-Living and Parasitic Stages of Cooperia Oncophora. Vet. Parasitol. 2017, 243, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.R.; Peña-Espinoza, M.A.; Boas, U.; Simonsen, H.T.; Enemark, H.L.; Thamsborg, S.M. Anthelmintic Activity of Chicory (Cichorium Intybus): In Vitro Effects on Swine Nematodes and Relationship to Sesquiterpene Lactone Composition. Parasitology 2016, 143, 770–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.G.; Cassida, K.A.; Turner, K.E. In Vitro Analysis of the Anthelmintic Activity of Forage Chicory (Cichorium Intybus L.) Sesquiterpene Lactones against a Predominantly Haemonchus Contortus Egg Population. Vet. Parasitol. 2011, 180, 298–306. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Thamsborg, S.M.; Desrues, O.; Hansen, T.V.A.; Enemark, H.L. Anthelmintic Effects of Forage Chicory (Cichorium Intybus) against Gastrointestinal Nematode Parasites in Experimentally Infected Cattle. Parasitology 2016, 143, 1279–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzamaloukas, O.; Athanasiadou, S.; Kyriazakis, I.; Huntley, J.F.; Jackson, F. The Effect of Chicory (Cichorium Intybus) and Sulla (Hedysarum Coronarium) on Larval Development and Mucosal Cell Responses of Growing Lambs Challenged with Teladorsagia Circumcincta. Parasitology 2006, 132, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Tzamaloukas, O.; Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. The Consequences of Short-Term Grazing of Bioactive Forages on Established Adult and Incoming Larvae Populations of Teladorsagia Circumcincta in Lambs. Int. J. Parasitol. 2005, 35, 329–335. [Google Scholar] [CrossRef]
- Kidane, A.; Parissi, Z.M.; Houdijk, J.G.M.; Athanasiadou, S.; Tolkamp, B.J.; Kyriazakis, I. Chicory (Cichorium Intybus) as Promising Bioactive Forage for Sheep Production Feed-a-Gene View Project COST Action CA16208 for Enhancing Management of European Riparian Ecosystems and Services View Project. Grassl. Sci. Eur. 2009, 14, 394–397. [Google Scholar]
- Webb, N.R. The Traditional Management of European Heathlands. J. Appl. Ecol. 1998, 35, 987–990. [Google Scholar] [CrossRef]
- Celaya, R.; Oliván, M.; Ferreira, L.M.M.; Martínez, A.; García, U.; Osoro, K. Comparison of Grazing Behaviour, Dietary Overlap and Performance in Non-Lactating Domestic Ruminants Grazing on Marginal Heathland Areas. Livest. Sci. 2007, 106, 271–281. [Google Scholar] [CrossRef]
- Moreno-Gonzalo, J.; Osoro, K.; García, U.; Frutos, P.; Celaya, R.; Ferreira, L.M.M.; Ortega-Mora, L.M.; Ferre, I. Anthelmintic Effect of Heather in Goats Experimentally Infected with Trichostrongylus Colubriformis. Parasitol. Res. 2014, 113, 693–699. [Google Scholar] [CrossRef]
- Osoro, K.; Celaya, R.; Moreno-Gonzalo, J.; Ferreira, L.M.M.; García, U.; Frutos, P.; Ortega-Mora, L.M.; Ferre, I. Effects of Stocking Rate and Heather Supplementation on Gastrointestinal Nematode Infections and Host Performance in Naturally-Infected Cashmere Goats. Rangel. Ecol. Manag. 2009, 62, 127–135. [Google Scholar] [CrossRef]
- Celaya, R.; Ferreira, L.M.M.; Moreno-Gonzalo, J.; Frutos, P.; Hervás, G.; Ferre, I.; García, U.; Ortega-Mora, L.M.; Osoro, K. Effects of Heather and Oat Supplementation on Gastrointestinal Nematode Infections and Performance of Grazing Cashmere Goats. Small Rumin. Res. 2010, 91, 186–192. [Google Scholar] [CrossRef]
- Osoro, K.; Benito-Peña, A.; Frutos, P.; García, U.; Ortega-Mora, L.M.; Celaya, R.; Ferre, I. The Effect of Heather Supplementation on Gastrointestinal Nematode Infections and Performance in Cashmere and Local Celtiberic Goats on Pasture. Small Rumin. Res. 2007, 67, 184–191. [Google Scholar] [CrossRef]
- Osoro, K.; Oliván, M.; Celaya, R.; Martínez, A. Effects of Genotype on the Performance and Intake Characteristics of Sheep Grazing Contrasting Hill Vegetation Communities. Anim. Sci. 1999, 69, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Ruisi, P.; Siragusa, M.; Di Giorgio, G.; Graziano, D.; Amato, G.; Carimi, F.; Giambalvo, D. Pheno-Morphological, Agronomic and Genetic Diversity among Natural Populations of Sulla (Hedysarum Coronarium L.) Collected in Sicily, Italy. Genet. Resour. Crop Evol. 2011, 58, 245–257. [Google Scholar] [CrossRef]
- Burke, J.L.; Waghorn, G.C.; Brookes, I.M. An Evaluation of Sulla (Hedysarum Coronarium) with Pasture, White Clover and Lucerne for Lambs; New Zealand Society of Animal Production: Palmerston North, New Zealand, 2002; Volume 62, pp. 152–156. [Google Scholar]
- Bonanno, A.; Di Grigoli, A.; Miceli, G.; Giambalvo, D. Grazing Sulla and/or Ryegrass Forage for 8 or 24 Hours Daily. Effects on Ewes Feeding Behaviour. In Proceedings of the Permanent and Temporary Grassland Plant, Environment and Economy, Ghent, Belgium, 3–5 September 2007. [Google Scholar]
- Molle, G.; Decandia, M.; Giovanetti, V.; Cabiddu, A.; Fois, N.; Sitzia, M. Responses to Condensed Tannins of Flowering Sulla (Hedysarum Coronarium L.) Grazed by Dairy Sheep: Part 1: Effects on Feeding Behaviour, Intake, Diet Digestibility and Performance. Livest. Sci. 2009, 123, 138–146. [Google Scholar] [CrossRef]
- Bonanno, A.; Di Miceli, G.; Di Grigoli, A.; Frenda, A.S.; Tornambè, G.; Giambalvo, D.; Amato, G. Effects of Feeding Green Forage of Sulla (Hedysarum Coronarium L.) on Lamb Growth and Carcass and Meat Quality. Animal 2011, 5, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Bonanno, A.; Di Grigoli, A.; Stringi, L.; Di Miceli, G.; Giambalvo, D.; Tornambè, G.; Vargetto, D.; Alicata, M.L. Intake and Milk Production of Goats Grazing Sulla Forage under Different Stocking Rates. Ital. J. Anim. Sci. 2016, 6, 605–607. [Google Scholar] [CrossRef]
- Burke, J.L.; Brookes, I.M.; McNabb, W.C.; Waghorn, G.C. The Potential of Sulla in Pasture-Based Systems. Sci. Access 2004, 1, 25–28. [Google Scholar]
- Amato, G.; Di Miceli, G.; Giambalvo, D.; Scarpello, C.; Stringi, L. Condensed Tannins Content in Sulla (Hedysarum Coronarium L.) as Affected by Environment, Genotype and Growth Stage. In Bioactive Compounds in Pasture Species for Phytotherapy and Animal Welfare; CNR-ISPAAM: Sassari, Italy, 2005; pp. 41–54. [Google Scholar]
- Pomroy, W.E.; Adlington, B.A. Efficacy of Short-Term Feeding of Sulla (Hedysarum Coronarium) to Young Goats against a Mixed Burden of Gastrointestinal Nematodes. Vet. Parasitol. 2006, 136, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Waghorn, G.C.; Min, B.R.; Mcnabb, W.C. The Effect of Condensed Tannins from Seven Herbages on Trichostrongylus Colubriformis Larval Migration in Vitro. Folia Parasitol. 2000, 47, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neizen, J.H.; Waghorn, T.S.; Waghorn, G.C.; Charleston, W.A.G. Internal Parasites and Lamb Production: A Role for Plants Containing Condensed Tannins? New Zealand Society of Animal Production: Palmerston North, New Zealand; Volume 53, p. 235. 1993. [Google Scholar]
- Niezen, J.J.; Waghorn, T.S.; Raufaut, K.; Robertson, H.A. Lamb Weight Gain and Faecal Egg Count When Grazing One of Seven Herbages and Dosed with Larvae for Six Weeks; New Zealand Society of Animal Production: Palmerston North, New Zealand, 1994; Volume 54, p. 15. [Google Scholar]
- Robertson, H.A.; Niezen, J.H.; Waghorn, G.C.; Charleston, W.A.G. The Effect of Six Herbages on Liveweight Gain, Wool Growth and Faecal Egg Count of Parasitised Ewe Lambs; New Zealand Society of Animal Production: Palmerston North, New Zealand, 1995; Volume 55, p. 199. [Google Scholar]
- Feedipedia Feedipedia: An on-Line Encyclopedia of Animal Feeds|Feedipedia. Available online: https://www.feedipedia.org/ (accessed on 19 September 2022).
- D’Addabbo, T.; Tava, A.; Argentieri, M.P.; Biazzi, E.; Candido, V.; Avato, P. Nematicidal Potential of Sulla (Hedysarum Coronarium L.) against the Root-Knot Nematode Meloidogyne Incognita. Plants 2022, 11, 2550. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, G.C.; Shelton, I.D. Effect of Condensed Tannins in Lotus Corniculatus on the Nutritive Value of Pasture for Sheep. J. Agric. Sci. 1997, 128, 365–372. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.A.; Barry, T.N.; López-Villalobos, N.; Kemp, P.D.; McNabb, W.C. Use of Lotus Corniculatus Containing Condensed Tannins to Increase Lamb and Wool Production under Commercial Dryland Farming Conditions without the Use of Anthelmintics. Anim. Feed Sci. Technol. 2004, 117, 85–105. [Google Scholar] [CrossRef]
- Cireșan, C.-A.; Oprescu, I.; Imre, M.; Suici, T.; Carpinișan, L.; Dărăbuș, G. Preliminary Research on the Control of Gastrointestinal Strongyles in Sheep, Using Lotus Corniculatus. Sci. Parasitol. 2021, 22, 44–49. [Google Scholar]
- Acharya, J.; Hildreth, M.B.; Reese, R.N. In Vitro Screening of Forty Medicinal Plant Extracts from the United States Northern Great Plains for Anthelmintic Activity against Haemonchus Contortus. Vet. Parasitol. 2014, 201, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Niezen, J.H.; Waghorn, G.C.; Charleston, W.A.G. Establishment and Fecundity of Ostertagia Circumcincta and Trichostrongylus Colubriformis in Lambs Fed Lotus (Lotus Pedunculatus) or Perennial Ryegrass (Lolium Perenne). Vet. Parasitol. 1998, 78, 13–21. [Google Scholar] [CrossRef]
- Mata-Padrino, D.J.; Belesky, D.P.; Crawford, C.D.; Walsh, B.; MacAdam, J.W.; Bowdridge, S.A. Effects of Grazing Birdsfoot Trefoil–Enriched Pasture on Managing Haemonchus Contortus Infection in Suffolk Crossbred Lambs. J. Anim. Sci. 2019, 97, 172–183. [Google Scholar] [CrossRef]
- Saratsis, A.; Voutzourakis, N.; Theodosiou, T.; Stefanakis, A.; Sotiraki, S. The Effect of Sainfoin (Onobrychis Viciifolia) and Carob Pods (Ceratonia Siliqua) Feeding Regimes on the Control of Lamb Coccidiosis. Parasitol. Res. 2016, 115, 2233–2242. [Google Scholar] [CrossRef]
- Hayot Carbonero, C.; Mueller-Harvey, I.; Brown, T.A.; Smith, L. Sainfoin (Onobrychis Viciifolia): A Beneficial Forage Legume. Plant Genet. Resour. 2011, 9, 70–85. [Google Scholar] [CrossRef]
- Heckendorn, F.; Häring, D.A.; Maurer, V.; Langhans, W.; Hertzberg, H. Effect of Sainfoin (Onobrychis Viciifolia) Silage and Hay against Gastrointestinal Nematodes in Lambs. In Proceedings of the Zwischen Tradition und Globalisierung—9. Wissenschaftstagung Ökologischer Landbau, Stuttgart, Germany, 20–23 March 2007. [Google Scholar]
- Novobilský, A.; Stringano, E.; Hayot Carbonero, C.; Smith, L.M.J.; Enemark, H.L.; Mueller-Harvey, I.; Thamsborg, S.M. In Vitro Effects of Extracts and Purified Tannins of Sainfoin (Onobrychis Viciifolia) against Two Cattle Nematodes. Vet. Parasitol. 2013, 196, 532–537. [Google Scholar] [CrossRef]
- Lane, G.P.F.; Koivisto, J.M. A Re-Assessment of the Potential of Sainfoin (Onobrychis Viciifolia Scop.) as a Forage Crop for the United Kingdom. In Proceedings of the 37th North American Alfalfa Improvement Conference, Madison, WI, USA, 16–19 July 2000; pp. 202–205. [Google Scholar]
- Regos, I.; Urbanella, A.; Treutter, D. Identification and Quantification of Phenolic Compounds from the Forage Legume Sainfoin (Onobrychis Viciifolia). J. Agric. Food Chem. 2009, 57, 5843–5852. [Google Scholar] [CrossRef] [PubMed]
- Thamsborg, S.M.; Mejer, H.; Bandier, M.; Larsen, M. Influence of Different Forages on Gastrointestinal Namatode Infections in Grazing Lambs. In Proceedings of the 19th International Conference of the World Association for the Advancement of Veterinary Parasitology, New Orleans, LA, USA, 10–14 August 2003. [Google Scholar]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F. The Effects of Feeding Sainfoin Hay in Sheep Parasitised with Trichostrongylus Colubriformis. Proc. Br. Soc. Anim. Sci. 2005, 2005, 90. [Google Scholar] [CrossRef]
- Collas, C.; Sallé, G.; Dumont, B.; Cabaret, J.; Cortet, J.; Martin-Rosset, W.; Wimel, L.; Fleurance, G. Are Sainfoin or Protein Supplements Alternatives to Control Small Strongyle Infection in Horses? Animal 2018, 12, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Stringano, E.; Hayot Carbonero, C.; Smith, L.M.J.; Brown, R.H.; Mueller-Harvey, I. Proanthocyanidin Diversity in the EU ‘HealthyHay’ Sainfoin (Onobrychis Viciifolia) Germplasm Collection. Phytochemistry 2012, 77, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Werne, S.; Isensee, A.; Maurer, V.; Perler, E.; Drewek, A.; Heckendorn, F. Integrated Control of Gastrointestinal Nematodes in Lambs Using a Bioactive Feed × Breed Approach. Vet. Parasitol. 2013, 198, 298–304. [Google Scholar] [CrossRef]
- Brunet, S.; Fourquaux, I.; Hoste, H. Ultrastructural Changes in the Third-Stage, Infective Larvae of Ruminant Nematodes Treated with Sainfoin (Onobrychis Viciifolia) Extract. Parasitol. Int. 2011, 60, 419–424. [Google Scholar] [CrossRef]
- Desrues, O.; Mueller-Harvey, I.; Pellikaan, W.F.; Enemark, H.L.; Thamsborg, S.M. Condensed Tannins in the Gastrointestinal Tract of Cattle after Sainfoin (Onobrychis Viciifolia) Intake and Their Possible Relationship with Anthelmintic Effects. J. Agric. Food Chem. 2017, 65, 1420–1427. [Google Scholar] [CrossRef]
- Legendre, H.; Hoste, H.; Gidenne, T. Nutritive Value and Anthelmintic Effect of Sainfoin Pellets Fed to Experimentally Infected Growing Rabbits. Animal 2017, 11, 1464–1471. [Google Scholar] [CrossRef] [Green Version]
- Azuhnwi, B.N.; Hertzberg, H.; Arrigo, Y.; Gutzwiller, A.; Hess, H.D.; Mueller-Harvey, I.; Torgerson, P.R.; Kreuzer, M.; Dohme-Meier, F. Investigation of Sainfoin (Onobrychis Viciifolia) Cultivar Differences on Nitrogen Balance and Fecal Egg Count in Artificially Infected Lambs. J. Anim. Sci. 2013, 91, 2343–2354. [Google Scholar] [CrossRef] [Green Version]
- Ríos-De Álvarez, L.; Greer, A.W.; Jackson, F.; Athanasiadou, S.; Kyriazakis, I.; Huntley, J.F. The Effect of Dietary Sainfoin (Onobrychis Viciifolia) on Local Cellular Responses to Trichostrongylus Colubriformis in Sheep. Parasitology 2008, 135, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Rivaroli, D.; Prunier, A.; Meteau, K.; Do Prado, I.N.; Prache, S. Tannin-Rich Sainfoin Pellet Supplementation Reduces Fat Volatile Indoles Content and Delays Digestive Parasitism in Lambs Grazing Alfalfa. Animal 2019, 13, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fajardo, R.A.; González-Pech, P.G.; Sandoval-Castro, C.A.; de Jesús Torres-Acosta, J.F. Small Ruminant Production Based on Rangelands to Optimize Animal Nutrition and Health: Building an Interdisciplinary Approach to Evaluate Nutraceutical Plants. Animals 2020, 10, 1799. [Google Scholar] [CrossRef] [PubMed]
| In Vivo/In Vitro | Animal Species | Indoor/Outdoor | Natural Infection/Experimental Infection | Parasites | Specific Parasite | Dosage | References |
|---|---|---|---|---|---|---|---|
| In vitro | - | - | - | Coccidia | Cryptosporidium parvum | 300, 150, 75, 37.5, 18.75 and 9.375 μg/mL | [162] |
| GIN | Ostertagia ostertagi | 1000, 500, 250, 100, 50 and 10 µg/mL | [82] | ||||
| Teladorsagia circumcincta, Cooperia oncophora and Ascaris suum | 7.8 and 500 μg/mL; 1 mg/mL | [130] | |||||
| Caenorhabditis elegans and A. suum | 15.6 to 20,000 μg/mL | [161] | |||||
| C. oncophora | Egg hatch assay: 2500, 1250, 625, 313 and 156 μg/mL Adult motility inhibition assay: 1000, 500, 250, 125, 60 and 30 μg/mL | [163] | |||||
| A. suum and Oesophagostomum dentatum | - | [164] | |||||
| Haemonchus contortus | 1.67–10.03 mg/mL | [165] | |||||
| Sheep | - | - | GIN | H. contortus, Trichostrongylus species, Trichostrongylus axei, Strongyloides and Bunstomum | 12.5, 25 and 50 mg/mL | [148] | |
| In vivo | Cattle | Indoor | Experimental infection | GIN | O. ostertagi and C. oncophora | ad libitum | [166] |
| Outdoor | Experimental infection | GIN | O. ostertagi | ad libitum | [166] | ||
| Deer | Outdoor | Natural infection | GIN | - | 0.17 and 0.26% DM | [134] | |
| Lung worms | - | 0.17 and 0.26% DM | [134] | ||||
| Sheep | - | Experimental infection | GIN | Ostertagia, Trichostrongylus, Oesophagostomu, Cooperia, and Nematodirus spp. | 2 or 4 kg DM/head per day (green) | [158] | |
| Natural infection | GIN | H. contortus, Trichostrongylus species, T. axei, Strongyloides and Bunstomum | 12.5, 25 and 50 mg/mL | [148] | |||
| Indoor | Experimental infection | GIN | H. contortus and Cooperia curticei | 3.4 g/kg DM | [60] | ||
| Outdoor | Experimental infection | GIN | Trichostrongylus colubriformis | 1.49 kg DM/day | [125] | ||
| T. circumcincta | 8.3 g/kg DM | [167] | |||||
| T. circumcincta | total phenolic: 262 g/kg DM | [168] | |||||
| Natural infection | GIN | T. circumcincta, Trichostrongylus vitrinus, T. axei, C. oncophora and Nematodirus battus. | total phenolic: 18 and 27 g/kg DM | [33] | |||
| T. circumcincta | - | [71] | |||||
| T. circumcita, H. contortus, C. curticei, Trichostrongylus spp., Chabertia ovina, Oesophagostomum spp. | - | [159] | |||||
| - | - | [160,169] |
| Unit | Average | |
|---|---|---|
| Crude protein | %DM | 24.3 |
| Crude fibre | %DM | 16.9 |
| Lignin | %DM | 0.33 |
| Ash | %DM | 18.8 |
| Condensed tannins | %DM | 0.17 |
| Sesquiterpene lactones | %DM | 0.36 |
| In Vivo/In Vitro | Animal Species | Indoor/Outdoor | Natural Infection/Experimental Infection | Parasites | Specific Parasite | Dosage | References |
|---|---|---|---|---|---|---|---|
| In vitro | Goat | - | - | GIN | Teladorsagia circumcincta and Haemonchus contortus | EC50: 450 μg/mL | [54] |
| H. contortus and Trichostrongylus colubriformis | 19% DM | [73] | |||||
| T. colubriformis | EC50: 120.9, 335.7, 521.6 and 791.3 μg/mL | [118] | |||||
| In vivo | Goat | Indoor | Experimental infection | GIN | T. circumcincta | 48.2 g tannin acid equivalent/kg DM | [68] |
| T. colubriformis | 64 g tannin acid equivalent/kg DM | [172] | |||||
| Outdoor | Natural infection | GIN | Trichostrongylus, Teladorsagia and Oesophagostomum | 64 g/kg DM | [105] | ||
| T. circumcincta, Trichostrongylus spp., and Chabertia ovina. | 7–8.6% DM | [132] | |||||
| Trichostrongylus spp., T. circumcincta, Oesophagostomum columbianum, Chabertia ovina and H. contortus | 84 g tannin acid equivalent/kg DM | [173] | |||||
| Teladorsagia, Trichostrongylus and Oesophagostomum genera | 30.2–47.2 g tannin acid equivalent/kg DM | [174] | |||||
| T. circumcincta, Teladorsagia trifurcata, Trichostrongylus axei, Trichostrongylus spp., C. ovina, O. columbianum, Trichuris ovis | 61–97 g tannin acid equivalent/kg DM | [175] | |||||
| Horse | Outdoor | Natural infection | GIN | - | - | [136] |
| Unit | Average | SD | |
|---|---|---|---|
| Dry matter | % as fed | 12.3 | 2.5 |
| Crude protein | %DM | 20.2 | 3.1 |
| Crude fibre | %DM | 24.3 | 4.1 |
| NDF | %DM | 36.8 | 5.8 |
| ADF | %DM | 28.8 | 5.4 |
| Lignin | %DM | 8.5 | 2.0 |
| Ether extract | %DM | 2.5 | 0.4 |
| Ash | %DM | 11.4 | 1.7 |
| Starch | %DM | 2.4 | |
| Condensed tannins [191] | %DM | 11.97 | 0.43 |
| In Vivo/In Vitro | Animal Species | Indoor/Outdoor | Natural Infection/Experimental Infection | Parasites | Specific Parasite | Dosage | References |
|---|---|---|---|---|---|---|---|
| In vitro | - | - | - | GIN | Ostertagia ostertagi or Cooperia oncophora | 600, 1200, 1400 μg/mL | [74] |
| Haemonchus contortus | EC50: 0.66–9.36 mg/mL EC90: 1.48–97.15 mg/mL | [76] | |||||
| H. contortus | 50 mg/mL | [195] | |||||
| Sheep | - | - | GIN | Trichostrongylus colubriformis | 1.6 and 5.5% DM | [135] | |
| In vivo | Deer | Indoor | Experimental infection | GIN | - | 1.9% DM | [117] |
| Lung worms | Dictyocaulus sp | 1.9% DM | [117] | ||||
| Sheep | Indoor | Experimental infection | GIN | H. contortus and Cooperia curticei | 15.2 g/kg DM | [60] | |
| H. contortus | 1.8–1.93 kg DM/animal day | [83] | |||||
| T. colubriformis | 1.6 and 5.5% DM | [135] | |||||
| Ostertagia circumcincta and T. colubriformis | - | [187] | |||||
| O. circumcincta and T. colubriformis | ad libitum | [196] | |||||
| Natural infection | GIN | - | ad libitum | [194] | |||
| Outdoor | Experimental infection | GIN | T. colubriformis | 15.9 g/kg DM | [125] | ||
| Teladorsagia circumcincta | 16 g/kg DM | [168] | |||||
| H. contortus | 13.3–17.4% DM | [197] | |||||
| Natural infection | GIN | Ostertagia, Haemonchus, Trichostrongylus, Nematodirus, Cooperia | - | [137] | |||
| T. circumcita, H. contortus, C. curticei, Trichostrongylus spp., Chabertia ovina, Oesophagostomum spp. | - | [159] | |||||
| - | - | [160] | |||||
| - | 25 g/kg DM | [193] |
| Unit | Average | SD | |
|---|---|---|---|
| Dry matter | % as fed | 23.1 | 6.8 |
| Crude protein | %DM | 21.1 | 4.2 |
| Crude fibre | %DM | 26.4 | |
| NDF | %DM | 38.3 | 8.14 |
| ADF | %DM | 28.2 | 5.5 |
| Lignin | %DM | 9.9 | 3.5 |
| Ether extract | %DM | 4.1 | 0.8 |
| Ash | %DM | 9.6 | 1.9 |
| Condensed tannins | %DM | 4.96 | 2.7 |
| Unit | Average | SD | |
|---|---|---|---|
| Dry matter | % as fed | 22.3 | 3.6 |
| Crude protein | %DM | 16.9 | 2.7 |
| Crude fibre | %DM | 25.8 | 4.9 |
| NDF | %DM | 35.4 | 5.7 |
| ADF | %DM | 30.1 | 4.0 |
| Lignin | %DM | 9.4 | 1.3 |
| Ether extract | %DM | 4.1 | 0.2 |
| Ash | %DM | 8.0 | 1.2 |
| Condensed tannins | %DM | 3.0 |
| In Vivo/In Vitro | Animal Species | Indoor/Outdoor | Natural Infection/Experimental Infection | Parasites | Specific Parasite | Dosage | References |
|---|---|---|---|---|---|---|---|
| In vitro | - | - | - | GIN | Ostertagia ostertagi or Cooperia oncophora | 600, 1200, 1400 μg/mL | [74] |
| Cattle | - | - | GIN | C. oncophora and O. ostertagi | 10, 40 μg/mL | [201] | |
| Deer | - | - | GIN | 1200 μg/mL | [78] | ||
| Lung worms | Dictyocaulus stages viviparus | 1200 μg/mL | [78] | ||||
| Goat | - | - | GIN | Haemonchus contortus (mainly) and Teladorsagia circumcincta | 1.2 lg/mL | [37,52,209] | |
| H. contortus and Trichostrongylus colubriformis | 1200 μg/mL | [77] | |||||
| T. circumcincta, H. contortus and T. colubriformis | 1200 μg/mL | [79] | |||||
| Horse | - | - | GIN | - | - | [206] | |
| Sheep | - | - | Coccidia | Eimeria crandallis | 150, 300, 1200 μg/mL | [61] | |
| GIN | T. colubriformis | 200, 400 μg/mL | [72,186] | ||||
| T. circumcincta, H. contortus and T. colubriformis | 1200 μg/mL | [79] | |||||
| H. contortus | - | [126] | |||||
| T. colubriformis | 400, 800 and 1000 μg/mL | [186] | |||||
| In vivo | Cattle | Indoor | Experimental infection | GIN | O. ostertagi and C. oncophora | - | [2] |
| 2.3% DM | [210] | ||||||
| Goat | Indoor | Experimental infection | GIN | H. contortus | 3.5% DM | [69] | |
| Natural infection | GIN | Trichostrongyle | [70] | ||||
| Outdoor | Natural infection | GIN | H. contortus, T. circumcincta and T. colubriformis | 1.5 kg sainfoin hay/day | [138] | ||
| Horse | Indoor | Natural infection | GIN | - | - | [206] | |
| Rabbit | Indoor | Experimental infection | GIN | T. colubriformis | 1.8 g/day | [211] | |
| Sheep | - | Experimental infection | Coccidia | E. crandallis | 150, 300, 1200 μg/mL | [61] | |
| Natural infection | Coccidia | E. crandallis | 150, 300, 1200 μg/mL | [61] | |||
| Indoor | Natural infection | Coccidia | Eimeria spp. | - | [198] | ||
| Experimental infection | GIN | H. contortus and T. colubriformis | - | [1] | |||
| H. contortus | 600, 1200 and 2400 μg/mL | [18] | |||||
| H. contortus and Cooperia curticei | 26.1 g/kg DM | [60] | |||||
| H. contortus and T. colubriformis | 1200 μg/mL | [77] | |||||
| H. contortus | [126] | ||||||
| H. contortus and C. curticei | 0.13% DM | [200] | |||||
| Haemonchus spp., Teladorsagia spp., Nema-todirus spp. and Trichostrongylus spp. | 59.71 and 106.62 g/animal day | [208] | |||||
| H. contortus | 8.1 and 9.7% DM | [212] | |||||
| T. colubriformis | 1.211 kg/day of sainfoin | [213] | |||||
| Natural infection | GIN | - | 15.1 g/kg DM | [214] | |||
| Outdoor | Experimental infection | GIN | T. colubriformis | 14.9 g/kg DM | [125] | ||
| Natural infection | Coccidia | - | 15.1 g/kg DM | [214] | |||
| GIN | - | 15.1 g/kg DM | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Hernández, P.; Reyes-Palomo, C.; Sanz-Fernández, S.; Rufino-Moya, P.J.; Zafra, R.; Martínez-Moreno, F.J.; Rodríguez-Estévez, V.; Díaz-Gaona, C. Antiparasitic Tannin-Rich Plants from the South of Europe for Grazing Livestock: A Review. Animals 2023, 13, 201. https://doi.org/10.3390/ani13020201
Rodríguez-Hernández P, Reyes-Palomo C, Sanz-Fernández S, Rufino-Moya PJ, Zafra R, Martínez-Moreno FJ, Rodríguez-Estévez V, Díaz-Gaona C. Antiparasitic Tannin-Rich Plants from the South of Europe for Grazing Livestock: A Review. Animals. 2023; 13(2):201. https://doi.org/10.3390/ani13020201
Chicago/Turabian StyleRodríguez-Hernández, Pablo, Carolina Reyes-Palomo, Santos Sanz-Fernández, Pablo José Rufino-Moya, Rafael Zafra, Francisco Javier Martínez-Moreno, Vicente Rodríguez-Estévez, and Cipriano Díaz-Gaona. 2023. "Antiparasitic Tannin-Rich Plants from the South of Europe for Grazing Livestock: A Review" Animals 13, no. 2: 201. https://doi.org/10.3390/ani13020201
APA StyleRodríguez-Hernández, P., Reyes-Palomo, C., Sanz-Fernández, S., Rufino-Moya, P. J., Zafra, R., Martínez-Moreno, F. J., Rodríguez-Estévez, V., & Díaz-Gaona, C. (2023). Antiparasitic Tannin-Rich Plants from the South of Europe for Grazing Livestock: A Review. Animals, 13(2), 201. https://doi.org/10.3390/ani13020201