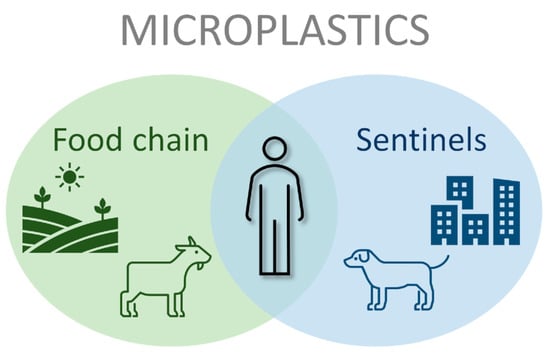
Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels
Abstract
:Simple Summary
Abstract
1. Introduction
2. Microplastics as a Food Security and Food Safety Threat on Livestock
3. The Role of Companion Animals as Sentinels for Environmental Exposure to Microplastics
4. Recommendations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Gaston, E.; Woo, M.; Steele, C.; Sukumaran, S.; Anderson, S. Microplastics Differ between Indoor and Outdoor Air Masses: Insights from Multiple Microscopy Methodologies. Appl. Spectrosc. 2020, 74, 1079–1098. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Sherri, M.; Wattenberg, E. Anthropogenic contamination of tap water, beer, and sea salt No Title. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One Health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Colpaert, R.; Petit dit Grézériat, L.; Louzon, M.; de Vaufleury, A.; Gimbert, F. Polyethylene microplastic toxicity to the terrestrial snail Cantareus aspersus: Size matters. Environ. Sci. Pollut. Res. 2022, 29, 29258–29267. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Carvalho, A.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Microplastics in Internal Tissues of Companion Animals from Urban Environments. Animals 2022, 12, 1979. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi, J.D.L.A.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef] [PubMed]
- Katsara, K.; Kenanakis, G.; Alissandrakis, E.; Papadakis, V.M. Low-Density Polyethylene Migration from Food Packaging on Cured Meat Products Detected by Micro-Raman Spectroscopy. Microplastics 2022, 1, 428–439. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Zhu, X.; Sun, C.; Teng, J.; Chen, L.; Shan, E.; Zhao, J. Microplastics in fish meals: An exposure route for aquaculture animals. Sci. Total Environ. 2022, 807, 151049. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Tolhurst, T.J.; Lindeque, P.K.; Thompson, R.; Cole, M. Detection and characterisation of microplastics and microfibres in fishmeal and soybean meal. Mar. Pollut. Bull. 2022, 185, 114189. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Tiruneh, R.; Yesuwork, H. Occurrence of rumen foreign bodies in sheep and goats slaughtered at the Addis Ababa Municipality Abattoir. Ethiop. Vet. J. 2011, 14, 91–100. [Google Scholar]
- Li, A.; Wang, Y.; Kulyar, M.F.-A.; Iqbal, M.; Lai, R.; Zhu, H.; Li, K. Environmental microplastics exposure decreases antioxidant ability, perturbs gut microbial homeostasis and metabolism in chicken. Sci. Total Environ. 2023, 856, 159089. [Google Scholar] [CrossRef]
- Susanti, R.; Yuniastuti, A.; Fibriana, F. The Evidence of Microplastic Contamination in Central Javanese Local Ducks from Intensive Animal Husbandry. Water Air Soil Pollut. 2021, 232, 178. [Google Scholar] [CrossRef]
- Leon, L.I.D.; Bautista, I.M.R.; Deza, A.G.M.D.; Kok, J.F.F.; Del Mundo, E.F.; VinceCruz-Abeledo, C.C. No Microplastic Fragments from Poultry Entrails in Wet Markets from South Caloocan, Philippines. Res. Sq. 2022; preprint. [Google Scholar]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Habib, R.Z.; Al Kindi, R.; Al Salem, F.; Kittaneh, W.F.; Poulose, V.; Iftikhar, S.H.; Mourad, A.-H.I.; Thiemann, T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. Int. J. Environ. Res. Public Health 2022, 19, 13442. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics contamination in eggs: Detection, occurrence and status. Food Chem. 2022, 397, 133771. [Google Scholar] [CrossRef]
- Wu, R.-T.; Cai, Y.-F.; Chen, Y.-X.; Yang, Y.-W.; Xing, S.-C.; Liao, X.-D. Occurrence of microplastic in livestock and poultry manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef]
- Habib, R.Z.; Poulose, V.; Alsaidi, R.; al Kendi, R.; Iftikhar, S.H.; Mourad, A.-H.I.; Kittaneh, W.F.; Thiemann, T. Plastic cutting boards as a source of microplastics in meat. Food Addit. Contam. Part A 2022, 39, 609–619. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Huerta Lwanga, E. Low density-microplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A.; et al. Detection and characterization of small-sized microplastics (≥5 µm) in milk products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhou, X.; Ding, W.; Wang, X.; Zhao, M.; Li, H.; Zou, G.; Chen, Y. Long-term application of organic compost is the primary contributor to microplastic pollution of soils in a wheat–maize rotation. Sci. Total Environ. 2022, 866, 161123. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.; Jiang, F.; Li, L.; Li, Y.; Zhang, M.; Qi, Z.; Ma, R.; Zhang, Y.; Fang, J.; et al. Detection of microplastics in domestic and fetal pigs’ lung tissue in natural environment: A preliminary study. Environ. Res. 2023, 216, 114623. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Origin of Synthetic Particles in Honeys. Polish J. Food Nutr. Sci. 2015, 65, 143–147. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501.
- Liao, Y.; Yang, J. The release process of Cd on microplastics in a ruminant digestion in-vitro method. Process Saf. Environ. Prot. 2022, 157, 266–272. [Google Scholar] [CrossRef]
- Quartinello, F.; Kremser, K.; Schoen, H.; Tesei, D.; Ploszczanski, L.; Nagler, M.; Podmirseg, S.M.; Insam, H.; Piñar, G.; Sterflingler, K.; et al. Together Is Better: The Rumen Microbial Community as Biological Toolbox for Degradation of Synthetic Polyesters. Front. Bioeng. Biotechnol. 2021, 9, 684459. [Google Scholar] [CrossRef]
- Raza, N.; Kim, K.-H. Quantification techniques for important environmental contaminants in milk and dairy products. TrAC Trends Anal. Chem. 2018, 98, 79–94. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Guo, J.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. The Association between Microplastics and Microbiota in Placentas and Meconium: The First Evidence in Humans. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef]
- Iulietto, M.F.; Evers, E.G. Modelling and magnitude estimation of cross-contamination in the kitchen for quantitative microbiological risk assessment (QMRA). EFSA J. 2020, 18, e181106. [Google Scholar]
- Cliver, D.O. Cutting Boards in Salmonella Cross-Contamination. J. AOAC Int. 2019, 89, 538–542. [Google Scholar]
- FDA Cutting Boards. Available online: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/cutting-boards (accessed on 2 January 2023).
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food Contact Materials. Available online: https://www.efsa.europa.eu/en/topics/topic/food-contact-materials (accessed on 8 June 2021).
- Prata, J.C.; Patrício Silva, A.L.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef]
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Rubio-Armendáriz, C.; Alejandro-Vega, S.; Paz-Montelongo, S.; Gutiérrez-Fernández, Á.J.; Carrascosa-Iruzubieta, C.J.; Hardisson-de la Torre, A. Microplastics as Emerging Food Contaminants: A Challenge for Food Safety. Int. J. Environ. Res. Public Health 2022, 19, 1174. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Prata, J.C.; Venâncio, C.; Girão, A.V.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of virgin and weathered polystyrene and polypropylene microplastics on Raphidocelis subcapitata and embryos of Danio rerio under environmental concentrations. Sci. Total Environ. 2022, 816, 151642. [Google Scholar] [CrossRef]
- Pulvirenti, E.; Ferrante, M.; Barbera, N.; Favara, C.; Aquilia, E.; Palella, M.; Cristaldi, A.; Conti, G.O.; Fiore, M. Effects of Nano and Microplastics on the Inflammatory Process: In Vitro and In Vivo Studies Systematic Review. Front. Biosci. 2022, 27, 287. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Garre, A.; Wiedmann, M.; Buchanan, R.L. All food processes have a residual risk, some are small, some very small and some are extremely small: Zero risk does not exist. Curr. Opin. Food Sci. 2021, 39, 83–92. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rilling, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Tallinen, S.; Mitrovic, S.M.; Penttinen, O.-P.; Kim, Y.-J.; Kim, S.; Esterhuizen, M. Case Study Comparing Effects of Microplastic Derived from Bottle Caps Collected in Two Cities on Triticum aestivum (Wheat). Environments 2021, 8, 64. [Google Scholar] [CrossRef]
- Potter, P.; Ramankutty, N.; Bennett, E.M.; Donner, S.D. Characterizing the Spatial Patterns of Global Fertilizer Application and Manure Production. Earth Interact. 2010, 14, 1–22. [Google Scholar] [CrossRef]
- Sun, Y.; Shaheen, S.M.; Ali, E.F.; Abdelrahman, H.; Sarkar, B.; Song, H.; Rinklebe, J.; Ren, X.; Zhang, Z.; Wang, Q. Enhancing microplastics biodegradation during composting using livestock manure biochar. Environ. Pollut. 2022, 306, 119339. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.; Liu, J.; Ren, X.; Zhang, Z.; Wang, Q. Effects of microplastics on humification and fungal community during cow manure composting. Sci. Total Environ. 2022, 803, 150029. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Li, R.; Hou, Y.; Chen, G.; Yan, B.; Mu, L. Effects of common microplastics on aerobic composting of cow manure: Physiochemical characteristics, humification and microbial community. J. Environ. Chem. Eng. 2022, 10, 108681. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, X.; Pan, J.; Zhang, Z.; Tsui, T.-H.; Luo, L.; Wang, Q. Effect of microplastics on greenhouse gas and ammonia emissions during aerobic composting. Sci. Total Environ. 2020, 737, 139856. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, X.; Zhang, J.; Cheng, P.; Xu, Z.; Sun, W.; Zhong, Y.; Wang, Y.; Yu, G.; Liu, H. Microplastics existence intensified bloom of antibiotic resistance in livestock feces transformed by black soldier fly. Environ. Pollut. 2023, 317, 120845. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef]
- Who Dietary Exposure Ietary Exposure Assessment Assessment of Chemicals of Chemicals in Food. Available online: https://apps.who.int/iris/bitstream/handle/10665/44027/9789241597470_eng.pdf;jsessionid=E1921D2CC7A96281222E381A46F96341?sequence=1 (accessed on 6 January 2023).
- Thushari, G.G.N.; Senevirathna, J.D.M.; Yakupitiyage, A.; Chavanich, S. Effects of microplastics on sessile invertebrates in the eastern coast of Thailand: An approach to coastal zone conservation. Mar. Pollut. Bull. 2017, 124, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Bråte, I.L.N.; Hurley, R.; Iversen, K.; Beyer, J.; Thomas, K.V.; Steindal, C.C.; Green, N.W.; Olsen, M.; Lusher, A. Mytilus spp. as sentinels for monitoring microplastic pollution in Norwegian coastal waters: A qualitative and quantitative study. Environ. Pollut. 2018, 243, 383–393. [Google Scholar] [CrossRef]
- Qu, X.; Su, L.; Li, H.; Liang, M.; Shi, H. Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci. Total Environ. 2018, 621, 679–686. [Google Scholar] [CrossRef]
- Al Malki, J.S.; Hussien, N.A.; Tantawy, E.M.; Khattab, Y.; Mohammadein, A. Terrestrial Biota as Bioindicators for Microplastics and Potentially Toxic Elements. Coatings 2021, 11, 1152. [Google Scholar] [CrossRef]
- Bordier, M.; Uea-Anuwong, T.; Binot, A.; Hendrikx, P.; Goutard, F.L. Characteristics of One Health surveillance systems: A systematic literature review. Prev. Vet. Med. 2020, 181, 104560. [Google Scholar] [CrossRef] [PubMed]
- Buttke, D.E. Toxicology, Environmental Health, and the “One Health” Concept. J. Med. Toxicol. 2011, 7, 329–332. [Google Scholar] [CrossRef]
- Neo, J.P.S.; Tan, B.H. The use of animals as a surveillance tool for monitoring environmental health hazards, human health hazards and bioterrorism. Vet. Microbiol. 2017, 203, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.L. Companion Animals as Sentinels for Public Health. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 241–250. [Google Scholar] [CrossRef]
- Beck, A.C.; Lash, E.M.; Hack, J.B. Environmental Toxic Exposures Using Companion Animals as an Indicator of Human Toxicity: A Case Report and Discussion. J. Emerg. Med. 2020, 59, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Kannan, K. Polyethylene Terephthalate and Polycarbonate Microplastics in Pet Food and Feces from the United States. Environ. Sci. Technol. 2019, 53, 12035–12042. [Google Scholar] [CrossRef]
- Prata, J.C. Strategies for the Improvement of Pet Health and Welfare in Portugal Based on a Pilot Survey on Husbandry, Opinion, and Information Needs. Animals 2020, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G. Gastrointestinal foreign bodies in dogs and cats: A retrospective study of 208 cases. J. Small Anim. Pract. 2009, 50, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Van Valkenburgh, B. The dog–human connection. Anat. Rec. 2021, 304, 10–18. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lo, P.-Y.; Wu, H.-D.; Chang, C.; Wang, L.-C. Association between indoor air pollution and respiratory disease in companion dogs and cats. J. Vet. Intern. Med. 2018, 32, 1259–1267. [Google Scholar] [CrossRef]
- Slack, J.D.; Kanke, M.; Simmons, G.H.; Deluca, P.P. Acute Hemodynamic Effects and Blood Pool Kinetics of Polystyrene Microspheres following Intravenous Administration. J. Pharm. Sci. 1981, 70, 660–664. [Google Scholar] [CrossRef]
- Ring, G.C.; Blum, A.S.; Kurbatov, T.; Moss, W.G.; Smith, W. Size of microspheres passing through pulmonary circuit in the dog. Am. J. Physiol. Content 1961, 200, 1191–1196. [Google Scholar] [CrossRef]
- Bartorelli, C.; Gerola, A. Tidal volume, oxygen uptake, cardiac output, and body surface in the cat. Am. J. Physiol. Content 1963, 205, 588–590. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef] [Green Version]

| Animal | Sample | Findings | Country | Reference |
|---|---|---|---|---|
| Duck | Intestine | 11–49 MP intestine−1 | Indonesia | [22] |
| Chicken | Crop | 11 MP crop−1 | Mexico | [13] |
| Gizzard | Present | Philippines | [23] | |
| 45.8 MP gizzard−1 | Mexico | [13] | ||
| Intestine | Present | Philippines | [23] | |
| Meat (packaged) | 4.0–18.7 MP kg−1 18–164 fibers kg−1 | France | [24] | |
| Meat (cut) | 30–1190 MP kg−1 | United Arab Emirates and Kuwaiti | [25] | |
| Eggs | 11.67 MP egg−1 | China | [26] | |
| Feces | 129,800 MP kg−1 | Mexico | [13] | |
| Manure | 667 MP kg−1 (w.w.) | China | [27] | |
| Goat | Meat (cut) | 2200–6500 MP kg−1 120–1620 mg kg−1 | Middle East | [28] |
| Sheep | Feces | 997 MP kg−1 | Spain | [29] |
| Cattle | Milk | 2040–10,040 MP L−1 | Switzerland and France | [30] |
| 3–11 MP L−1 | Mexico | [31] | ||
| 40 MP L−1 | Ecuador | [32] | ||
| Manure | 74 MP kg−1 (w.w.) | China | [27] | |
| 4520 MP kg−1 (d.w.) | China | [33] | ||
| Pig | Lungs | 180,000 MP kg−1 | China | [34] |
| Manure | 902 MP kg−1 (w.w.) | China | [27] | |
| 3547 MP kg−1 (d.w.) | China | [33] | ||
| Bee | Honey | 54 and 67 MP L−1 | Ecuador | [32] |
| 40–660 fibers kg−1 0–38 fragments kg−1 | Germany, France, Italy, Spain, Mexico | [19] | ||
| 10–336 fibers kg−1 2–86 fragments kg−1 | Germany | [35] |
| Product | Daily Intake (a) | Concentration Range (b) | Estimated Daily Intake (MP day−1) (c) |
|---|---|---|---|
| World Health Organization model | |||
| Meat | 300 g | 30–1190 MP kg−1 | 9–357 |
| Liver | 100 g | n.a. | n.a. |
| Kidney | 50 g | n.a. | n.a. |
| Animal fat | 50 g | n.a. | n.a. |
| Eggs | 100 g (d) | 12 MP egg−1 | 24 |
| Milk | 1.5 L | 3–10,000 MP L−1 | 5–15,000 |
| Europe Union trends (2013) | |||
| Poultry meat | 316 g | 30–1190 MP kg−1 | 10–376 |
| Dairy products | 286 g | 3–10,000 MP L−1 | 1–2766 |
| Pig Meat | 96 g | n.a. | n.a. |
| Bovine Meat | 86 g | n.a. | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prata, J.C.; Dias-Pereira, P. Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels. Animals 2023, 13, 661. https://doi.org/10.3390/ani13040661
Prata JC, Dias-Pereira P. Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels. Animals. 2023; 13(4):661. https://doi.org/10.3390/ani13040661
Chicago/Turabian StylePrata, Joana C., and Patrícia Dias-Pereira. 2023. "Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels" Animals 13, no. 4: 661. https://doi.org/10.3390/ani13040661
APA StylePrata, J. C., & Dias-Pereira, P. (2023). Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels. Animals, 13(4), 661. https://doi.org/10.3390/ani13040661