Geochemical Processes Controlling Ionic Composition of Water in the Catchments of Lakes Saana and Saanalampi in the Kilpisjärvi Area of North Scandinavia
Abstract
:1. Introduction
2. The Study Area
3. Material and Methods
4. Results and Discussion
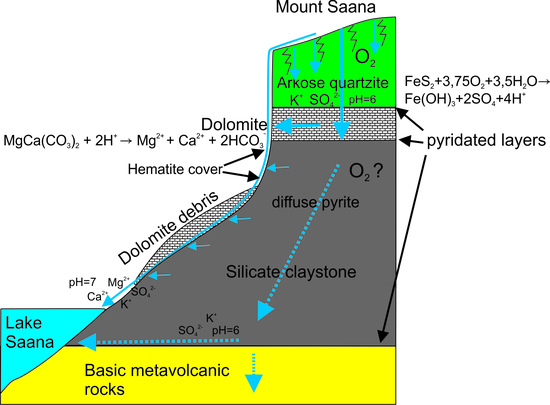
4.1. Rock and Soil Composition
4.2. Salinity Fluctuation in Space and Time
4.3. Carbonate Dissolution
4.4. Silicate Weathering
4.5. Stream Water Input into the Surroundings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berner, K.E.; Berner, R.A. Global Environment: Water, Air and Geochemical Cycles; Princeton University Press: Upper Saddle River, NJ, USA, 1996; 376p. [Google Scholar]
- Stutter, M.I.; Billett, M.F. Biogeochemical controls on streamwater and soil solution chemistry in a High Arctic environment. Geoderma 2003, 113, 127–146. [Google Scholar] [CrossRef]
- Lerman, A.; Wu, L.; Mackenzie, F.T. CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar. Chem. 2007, 106, 326–350. [Google Scholar] [CrossRef]
- Calmels, D.; Gaillardet, J.; François, L. Sensitivity of carbonate weathering to soil CO2 production by biological activity along a temperate climate transect. Chem. Geol. 2014, 390, 74–86. [Google Scholar] [CrossRef]
- Giesler, R.; Lyon, S.W.; Mörth, C.-M.; Karlsson, J.; Karlsson, E.M.; Jantze, E.J.; Destouni, G.; Humborg, C. Catchment-scale dissolved carbon concentrations and export estimates across six subarctic streams in northern Sweden. Biogeosciences 2014, 11, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Millot, R.; Gaillardet, J.; Dupré, B.; Allégre, C.J. Northern latitude chemical weathering rates: Clues from the Mackenzie River Basin, Canada. Geochim. Cosmochim. Acta 2003, 67, 1305–1329. [Google Scholar] [CrossRef]
- Thorn, C.E.; Darmody, R.G.; Dixon, J.C.; Schlyter, P. The chemical weathering regime of Kärkevagge, artic-alpine Sweden. Geomorphology 2001, 41, 37–52. [Google Scholar] [CrossRef]
- Darmody, R.G.; Thorn, C.E.; Harder, R.L.; Schlyter, J.P.L.; Dixon, J.C. Weathering implications of water chemistry in an arctic–alpine environment, northern Sweden. Geomorphology 2000, 34, 89–100. [Google Scholar] [CrossRef]
- Darmody, R.G.; Thorn, C.E.; Allen, C.E. Chemical weathering and boulder mantles, Kärkevagge, Swedish Lapland. Geomorphology 2005, 67, 159–170. [Google Scholar] [CrossRef]
- Beylich, A.A.; Kolstrup, E.; Linde, N.; Pedersen, L.B.; Thyrsted, T.; Gintz, D.; Dynesius, L. Assessment of chemical denudation rates using hydrological measurements, water chemistry analysis and electromagnetic geophysical data. Permafr. Periglac. Process. 2003, 14, 387–397. [Google Scholar] [CrossRef]
- Beylich, A.A.; Kneisel, C. Sediment budget and relief development in Hrafndalur, sub-Arctic oceanic eastern Iceland. Arct. Antarct. Alp. Res. 2009, 41, 3–17. [Google Scholar] [CrossRef]
- Dragon, K.; Marciniak, M. Chemical composition of groundwater and surface water in the Arctic environment (Petuniabukta region, central Spitsbergen). J. Hydrol. 2010, 386, 160–172. [Google Scholar] [CrossRef]
- Beylich, A.A. Mass transfers, sediment budgets and relief development in cold environments: Results of long-term geomorphologic drainage basin studies in Iceland, Swedish Lapland and Finnish Lapland. Zeitschrift für Geomorphologie N.F. 2011, 55, 145–174. [Google Scholar] [CrossRef]
- Beylich, A.A.; Laute, K. Spatial variations of surface water chemistry and chemical denudation in the Erdalen drainage basin, Nordfjord, western Norway. Geomorphology 2012, 167, 77–90. [Google Scholar] [CrossRef]
- Dixon, J.C.; Thorn, C.E. Chemical weathering and landscape development in mid-latitude alpine environments. Geomorphology 2005, 67, 127–145. [Google Scholar] [CrossRef]
- Reynolds, R.C., Jr. Clay mineral formation in an alpine environment. Clays Clay Miner. 1971, 19, 361–374. [Google Scholar] [CrossRef]
- Caine, N.; Thurman, E.M. Temporal and spatial variations in the solute content of an alpine stream, Colorado Front Range. Geomorphology 1990, 4, 55–72. [Google Scholar] [CrossRef]
- Dixon, J.C.; Darmody, R.G.; Schlyter, P.; Thorn, C.E. Preliminary investigation of geochemical process responses to potential environmental change in Kärkevagge, Northern Scandinavia. Geografiska Annaler 1995, 77A, 259–267. [Google Scholar] [CrossRef]
- Forsström, L.; Sorvari, S.; Rautio, M.; Sonninen, E.; Korhola, A. Changes in physical and chemical limnology and plankton during the Spring melt period in a subarctic Lake. Int. Rev. Hydrobiol. 2007, 92, 301–325. [Google Scholar] [CrossRef]
- Sorvari, S.; Rautio, M.; Korhola, A. Seasonal dynamics of the subarctic Lake Saanajärvi in Finnish Lapland. Verheissungen Int. Ver. Gesamten Limnol. 2000, 27, 507–512. [Google Scholar] [CrossRef]
- Molot, L.A.; Dillon, P.J.; Lazerte, B.D. Factors affecting alkalinity concentrations of streamwater during snowmelt in Central Ontario. Can. J. Fish. Aquat. Sci. 1989, 46, 1658–1666. [Google Scholar] [CrossRef]
- Bishop, K.; Pettersson, C. Organic carbon in the boreal spring flood from adjacent subcatchments. Environ. Int. 1996, 22, 535–540. [Google Scholar] [CrossRef]
- Moiseenko, T.; Kudrjavzeva, L.; Rodyshkin, I. The episodic acidification of small streams in the spring flood period of industrial polar region, Russia. Chemosphere 2001, 42, 45–50. [Google Scholar] [CrossRef]
- Petrone, K.; Buffam, I.; Laudon, H. Hydrologic and biotic control of nitrogen export during snowmelt: a combined conservative and reactive tracer approach. Water Resour. Res. 2007, 43, W06420. [Google Scholar] [CrossRef]
- Laudon, H.; Westling, O.; Bishop, K. Cause of pH decline in stream water during spring melt runoff in northern Sweden. Can. J. Fish. Aquat. Sci. 2000, 57, 1888–1900. [Google Scholar] [CrossRef]
- De Caritat, P.; Hall, G.; Gislason, S.; Belsey, W.; Braun, M.; Goloubeva, N.I.; Olsen, H.K.; Scheie, J.O.; Vaive, J.E. Chemical composition of arctic snow: concentration levels and regional distribution of major elements. Sci. Total Environ. 2005, 336, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Raidla, V.; Kaup, E.; Ivask, J. Factors affecting the chemical composition of snowpack in the Kilpisjärvi area of North Scandinavia. Atmos. Environ. 2015, 118, 211–218. [Google Scholar] [CrossRef]
- Korhola, A.; Sorvari, S.; Rautio, M.; Appleby, P.G.; Dearing, J.A.; Hu, Y.; Rose, N.; Lami, A.; Cameron, N.G. A multi-proxy analysis of climate impacts on the recent development of subarctic Lake Saanajärvi in Finnish Lapland. J. Paleolimnol. 2002, 28, 59–77. [Google Scholar] [CrossRef]
- Carminati, E. Incremental strain analysis using two generations of syntectonic coaxial fibres: an example from the Monte Marguareis Briançonnais Cover nappe (Ligurian Alps, Italy). J. Struct. Geol. 2001, 23, 1441–1456. [Google Scholar] [CrossRef]
- Anderson, S.P.; Drever, J.I.; Frost, C.D.; Holden, P. Chemical weathering in the foreland of a retreating glacier. Geochim. Cosmochim. Acta 2000, 64, 1173–1189. [Google Scholar] [CrossRef]
- Cooper, R.J.; Wadham, J.L.; Trantera, M.; Hodgkinsb, R.; Peters, N.E. Groundwater hydrochemistry in the active layer of the proglacial zone, Finsterwalderbreen, Svalbard. J. Hydrol. 2002, 269, 208–223. [Google Scholar] [CrossRef]
- Gaillardet, J.; Millot, R.; Dupré, B. Chemical denudation rates of the western Canadian orogenic belt: The Stikine terrane. Chem. Geol. 2003, 201, 257–259. [Google Scholar] [CrossRef]
- Spence, J.; Telmer, K. The role of sulfur in chemical weathering and atmospheric CO2 fluxes: evidence from major ions, δ13CDIC, and δ34SSO4 in rivers of the Canadian Cordillera. Geochim. Cosmochim. Acta 2005, 69, 5441–5458. [Google Scholar] [CrossRef]
- Beaulieu, E.; Goddéris, Y.; Labat, D.; Roelandt, C.; Calmels, D.; Gaillardet, J. Modeling of water-rock interaction in the Mackenzie basin: Competition between sulfuric and carbonic acids. Chem. Geol. 2011, 289, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, A. Basic climatological data on the Klipisjärvi area, NW Finnish Lapland. Kilpisjärvi Notes 1987, 10, 1–16. [Google Scholar]
- Drebs, A.; Nordlund, A.; Karlsson, P.; Helminen, J.; Rissanen, P. Climatological statistics of Finland 1971–2000. In Climatic Statistics of Finland 2002: 1; Finnish Meteorological Institute: Helsinki, Finland, 2002; p. 99. [Google Scholar]
- Atlas of Finland. Geology, 1: 200000 Geological map of Finland. National Board of Survey, Geographical Society of Finland, 1986. Folio 123–126. Available online: http://gtkdata.gtk.fi/Kalliopera/index.html (accessed on 14 March 2019).
- Männistö, M.K.; Tiirola, M.; Häggblom, M.M. Bacterial communities in Arctic fjelds of Finnish Lapland are stable but highly pH-dependent. FEMS Microbiol. Ecol. 2007, 59, 452–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohout, T.; Bućko, M.S.; Rasmus, K.; Leppäranta, M.; Matero, I. Non-Invasive Geophysical Investigation and Thermodynamic Analysis of a Palsa in Lapland, Northwest Finland. Permafr. Periglac. Process. 2014, 25, 45–52. [Google Scholar] [CrossRef] [Green Version]
- King, L.; Seppälä, M. Permafrost Thickness and Distribution in Finnish Lapland—Results of Geoelectrical Soundings. Polarforschung 1987, 57, 127–147. [Google Scholar]
- Vanhala, H.; Lintinen, P.; Ojala, A. Electrical resistivity study of permafrost on Ridnitšohkka fell in northwest Lapland, Finland. Geophysica 2009, 45, 103–118. [Google Scholar]
- Finnish Meteorological Institute. The Finnish Meteorological Institute’s open data. Available online: https://en.ilmatieteenlaitos.fi/open-data (accessed on 1 August 2018).
- Calmels, D.; Gaillardet, J.; Brenot, A.; France-Lanord, C. Sustained sulfide oxidation by physical erosion processes in the Mackenzie River basin: climatic persperctives. Geology 2007, 35, 1003–1006. [Google Scholar] [CrossRef]
- Szynkiewicz, A.; Borrok, D.M.; Skrzypek, G.; Rearick, M.S. Isotopic studies of the Upper and Middle Rio Grande. Part 1—Importance of sulfide weathering in the riverine sulfate budget. Chem. Geol. 2015, 411, 323–335. [Google Scholar] [CrossRef]
- Appelo, C.; Postma, D. Geochemistry, Groundwater and Pollution; Balkema: Rotterdam, The Netherlands, 2004; p. 683. [Google Scholar]
- Jacobson, A.D.; Blum, J.D.; Walter, L.M. Reconciling the elemental and Sr isotope composition of Himalayan weathering fluxes: Insights from the carbonate geochemistry of stream waters. Geochim. Cosmochim. Acta 2002, 66, 3417–3429. [Google Scholar] [CrossRef]
- Arendt, C.A.; Stevenson, E.I.; Aciego, S.M. Hydrologic controls on radiogenic Sr in meltwater from an alpine glacier system: Athabasca Glacier, Canada. Appl. Geochem. 2016, 69, 42–49. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Postgate, J.R. Ecology of the bacteria of the sulphur cycle with special reference to anoxic–oxic interface environments. Philos. Trans. R. Soc. Lond. 1982, 298, 543–561. [Google Scholar] [CrossRef]
- Wadham, J.L.; Bottrell, S.; Tranter, M.; Raiswell, R. Stable isotope evidence for microbial sulphate reduction at the bed of a polythermal high Arctic glacier. Earth Planet. Sci. Lett. 2004, 219, 341–355. [Google Scholar] [CrossRef]
- Brunner, B.; Bernasconi, S.M.; Kleikemper, J.; Schroth, M.H. A model for oxygen and sulfur isotope fractionation in sulfate during bacterial sulfate reduction processes. Geochim. Cosmochim. Acta 2005, 69, 4773–4785. [Google Scholar] [CrossRef]
- Geng, H.; Jiyeon, R.; Jung, H.-J.; Chung, H.; Ahn, K.-H.; Ro, C.-U. Single-particle characterization of summertime Arctic aerosols collected at Ny-Ålesund, Svalbard. Environ. Sci. Technol. 2010, 44, 2348–2353. [Google Scholar] [CrossRef]
- Dixon, J.C.; Thorn, C.E.; Darmody, R.G.; Campbell, S.W. Weathering rinds and rock coatings from an Arctic alpine environment, northern Scandinavia. Geol. Soc. Am. Bull. 2002, 114, 226–238. [Google Scholar] [CrossRef]
- White, A.F.; Schulz, M.S.; Vivit, D.V.; Blum, A.E.; Stonestrom, D.A.; Harden, J.W. Chemical weathering rates of a soil chronosequence on granitic alluvium: III. Hydrochemical evolution and contemporary solute fluxes and rates. Geochim. Cosmochim. Acta 2005, 69, 1975–1996. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Hodson, A.; Tranter, M.; Gurnell, A.; Clark, M.; Hagen, J.O. The hydrochemistry of Bayelva, a high Arctic proglacial stream in Svalbard. J. Hydrol. 2002, 257, 91–114. [Google Scholar] [CrossRef]
- Tranter, M.; Brown, G.H.; Raiswell, R.; Sharp, M.J.; Gurnell, A.M. A conceptual model of solute acquisition by alpine glacial meltwaters. J. Glaciol. 1993, 39, 573–581. [Google Scholar] [CrossRef]
- Brown, G.H. Glacier meltwater hydrochemistry. Appl. Geochem. 2002, 17, 855–883. [Google Scholar] [CrossRef]
- Williams, M.W.; Knauf, M.; Caine, N.; Liu, F.; Verplanck, P.L. Geochemistry and source waters of rock glacier outflow, Colorado Front Range. Permafr. Periglac. Process. 2006, 17, 13–33. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.P.; Drever, J.I.; Humphrey, N.F. Chemical weathering in glacial environments. Geology 1997, 25, 399–402. [Google Scholar] [CrossRef]
- Hall, K.; Thorn, C.E.; Matsuoka, N.; Prick, A. Weathering in cold regions: some thoughts and perspectives. Prog. Phys. Geogr. 2002, 26, 577–603. [Google Scholar] [CrossRef]
- Matsuoka, N.; Moriwaki, K.; Hirakawa, K. Field experiments on physical weathering and wind erosion in an Antarctic cold desert. Earth Surf. Process. Landf. 1998, 21, 687–699. [Google Scholar] [CrossRef]
- Anderson, S.P. Biogeochemistry of glacial landscape systems. Annu. Rev. Earth Planet. Sci. 2007, 35, 375–399. [Google Scholar] [CrossRef]
- Marra, K.R.; Madden, M.E.E.; Soreghan, G.S.; Hall, B.L. BET surface area distributions in polar stream sediments: Implications for silicate weathering in a cold-arid environment. Appl. Geochem. 2015, 52, 31–42. [Google Scholar] [CrossRef]
- Anderson, S.P.; Dietrich, W.E.; Brimhall, G.H. Weathering profiles, mass-balance analysis, and rates of solute loss: linkages between weathering and erosion in a small, steep catchment. Geol. Soc. Am. Bull. 2002, 114, 1143–1158. [Google Scholar] [CrossRef]
- Yde, J.C.; Riger-Kusk, M.; Christiansen, H.H.; Knudsen, N.T.; Umlum, O. Hydrochemical characteristics of bulk meltwater from an entire ablation season, Longyearbreen, Svalbard. J. Glaciol. 2008, 54, 259–272. [Google Scholar] [CrossRef]
- Klaminder, J.; Grip, H.; Mörth, C.-M.; Laudon, H. Carbon mineralization and pyrite oxidation in groundwater: Importance for silicate weathering in boreal forest soils and stream base-flow chemistry. Appl. Geochem. 2011, 26, 319–325. [Google Scholar] [CrossRef]
- Heidel, C.; Tichomirowa, M.; Junghans, M. The influence of pyrite grain size on the final oxygen isotope difference between sulphate and water in aerobic pyrite oxidation experiments. Isot. Environ. Health Stud. 2009, 45, 321–342. [Google Scholar] [CrossRef]
- Aquilina, L.; Ladouche, B.; Dörfliger, N. Recharge processes in karstic systems investigated through the correlation of chemical and isotopic composition of rain and spring-waters. Appl. Geochem. 2005, 20, 2189–2206. [Google Scholar] [CrossRef]
- Mueller, M.H.; Weingartner, R.; Alewell, C. Importance of vegetation, topography and flow paths for water transit times of base flow in alpine headwater catchments. Hydrol. Earth Syst. Sci. 2013, 17, 1661–1679. [Google Scholar] [CrossRef] [Green Version]
- Genty, D.; Labuhn, I.; Hoffmann, G.; Danis, P.A.; Mestre, O.; Bourges, F.; Wainer, K.; Massault, M.; Van Exter, S.; Régnier, E.; et al. Rainfall and cave water isotopic relationships in two South-France sites. Geochim. Cosmochim. Acta 2014, 131, 323–343. [Google Scholar] [CrossRef] [Green Version]
- Lenoir, J.; Gégout, J.-C.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.E.; Dullinger, S.; Pauli, H.; Willner, W.; Grytnes, J.-A.; et al. Cross-scale analysis of the region effect on vascular plant species diversity in southern and northern European mountain ranges. PLoS ONE 2010, 5, e15734. [Google Scholar] [CrossRef] [PubMed]
- Systra, Y.J. Bedrock and Quaternary sediment geochemistry and biodiversity in Eastern Fennoscandia and Estonia. For. Stud./Metsanduslikud Uurimused 2010, 53, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Kauhanen, H.O. Mountains of Kilpisjärvi host an abundance of threatened plants in Finnish Lapland. Bot. Pac. 2013, 2, 43–52. [Google Scholar] [CrossRef]
- Kumar, M.; Männistö, M.K.; van Elsas, J.D.; Nissinen, R.M. Plants impact structure and function of bacterial communities in Arctic soils. Plant Soil 2016, 399, 319–332. [Google Scholar] [CrossRef]












| No. | Sampling Point | Sample Type | Coordinates | Depth | LOI920 °C | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | Cl | S | F | ||||||||||
| (N°) | (E°) | m | wt% | |||||||||||||||||||||||||
| 1 | 1 | Subsurface | 69°03′16.8″ | 20°51′43.8″ | 0.5 | 6.5 | 62 | 0.63 | 14.2 | 6.0 | 0.1 | 2.1 | 3.5 | 2.9 | 1.6 | 0.3 | 0.03 | 0.04 | ||||||||||
| 2 | 3 | Subsurface | 69°02′49.2″ | 20°51′38.2″ | 0.1 | 6.4 | 61 | 0.71 | 13.7 | 6.9 | 0.1 | 2.1 | 4.0 | 2.9 | 1.5 | 0.2 | 0.02 | 0.04 | <0.3 | |||||||||
| 3 | 3 | Subsurface | 0.55 | 3.8 | 65 | 0.70 | 13.5 | 5.7 | 0.1 | 2.0 | 4.0 | 3.3 | 1.5 | 0.2 | 0.03 | 0.03 | <0.3 | |||||||||||
| 4 | 3 | Subsurface | 1.0 | 42.4 | 35 | 0.60 | 10.5 | 3.9 | 0.1 | 1.3 | 2.6 | 1.5 | 0.9 | 0.4 | 0.03 | 0.57 | <0.3 | |||||||||||
| 5 | 8 | Subsurface | 69°02′59.5″ | 20°51′51.8″ | 0.1 | 8.4 | 65 | 0.42 | 11.9 | 3.6 | 0.1 | 1.4 | 3.3 | 3.0 | 1.7 | 0.2 | 0.02 | 0.17 | <0.3 | |||||||||
| 6 | 8 | Subsurface | 0.55 | 10.7 | 63 | 0.64 | 11.1 | 4.8 | 0.1 | 1.8 | 3.1 | 2.2 | 1.4 | 0.3 | 0.03 | 0.16 | <0.3 | |||||||||||
| 7 | 9 | Subsurface | 69°03′09.7″ | 20°51′31.4″ | 0.5 | 6.9 | 64 | 0.64 | 13.4 | 6.1 | 0.2 | 1.6 | 2.3 | 1.9 | 2.7 | 0.2 | 0.02 | 0.04 | ||||||||||
| 8 | KIL-1 | Meta-sediment | 69°02′35.5″ | 20°52′03.6″ | 1.7 | 72 | 0.56 | 13.0 | 3.2 | 0.1 | 1.2 | 1.8 | 2.8 | 3.1 | 0.03 | 0.05 | ||||||||||||
| 9 | KIL-10 | Silicate claystone | 69°02′27.2″ | 20°52′42.0″ | 4.6 | 65 | 0.94 | 16.4 | 5.0 | 0.2 | 1.8 | 1.1 | 0.5 | 4.1 | 0.02 | 0.09 | ||||||||||||
| 10 | KIL-2 | Basaltic dyke | 69°02′23.5″ | 20°52′59.8″ | 1.3 | 46 | 1.94 | 15.1 | 17.1 | 0.3 | 5.5 | 8.7 | 2.3 | 1.1 | 0.07 | 0.03 | ||||||||||||
| No. | Sampling Point | Sample Type | Coordinates | Depth | As | Ba | Br | Ce | Cr | Cu | Ga | Ge | La | Mo | Nb | Ni | Pb | Rb | Sc | Se | Sr | Th | U | V | Y | Zn | Zr | |
| (N°) | (E°) | m | ppm | |||||||||||||||||||||||||
| 1 | 1 | Subsurface | 69°03′16.8″ | 20°51′43.8″ | 0.5 | 425 | ||||||||||||||||||||||
| 2 | 3 | Subsurface | 69°02′49.2″ | 20°51′38.2″ | 0.1 | <5 | 483 | 3 | 21 | 66 | 25 | 18 | <10 | 5 | <5 | 4 | 39 | 11 | 45 | 0 | <5 | 281 | 5 | <10 | 119 | 20 | 66 | 154 |
| 3 | 3 | Subsurface | 0.55 | <5 | 498 | 0 | 30 | 65 | 26 | 16 | <10 | 17 | <5 | 6 | 39 | 8 | 41 | 8 | <5 | 288 | 0 | <10 | 111 | 21 | 66 | 168 | ||
| 4 | 3 | Subsurface | 1.0 | <5 | 298 | 11 | 130 | 65 | 200 | 11 | <10 | 76 | <5 | 6 | 24 | 8 | 27 | 13 | <5 | 151 | 12 | <10 | 145 | 43 | 44 | 106 | ||
| 5 | 8 | Subsurface | 69°02′59.5″ | 20°51′51.8″ | 0.1 | <5 | 445 | 6 | 11 | 48 | 16 | 15 | <10 | 0 | <5 | 4 | 27 | 10 | 42 | 0 | <5 | 244 | 0 | <10 | 68 | 16 | 52 | 121 |
| 6 | 8 | Subsurface | 0.55 | <5 | 401 | 7 | 29 | 59 | 26 | 13 | <10 | 19 | <5 | 5 | 35 | 16 | 49 | 3 | <5 | 221 | 5 | <10 | 93 | 24 | 73 | 163 | ||
| 7 | 9 | Subsurface | 69°03′09.7″ | 20°51′31.4″ | 0.5 | 607 | ||||||||||||||||||||||
| 8 | KIL-1 | Meta-sediment | 69°02′35.5″ | 20°52′03.6″ | 727 | 51 | 47 | 6 | 12 | 27 | 9 | 26 | 81 | 234 | 1 | 56 | 20 | 40 | 324 | |||||||||
| 9 | KIL-10 | Silicate claystone | 69°02′27.2″ | 20°52′42.0″ | 701 | 125 | 76 | 16 | 15 | 39 | 15 | 55 | 138 | 49 | 18 | 67 | 41 | 67 | 318 | |||||||||
| 10 | KIL-2 | Basaltic dyke | 69°02′23.5″ | 20°52′59.8″ | 200 | 50 | 165 | 111 | 16 | 9 | 11 | 81 | 33 | 165 | 3 | 298 | 35 | 147 | 172 | |||||||||
| No. | Sampling Point | Date | Depth | Coordinates | pH | El.cond. | SO42− | Cl− | Na+ | K+ | Ca2+ | Mg2+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | (N°) | (E°) | μS·cm−1 | mg·L−1 | |||||||||
| 1 | 1 | 30 June 2010 | 0.5 | 69°03′16.8″ | 20°51′43.8″ | 6.3 | 47.8 | 6.5 | 0.7 | 1.4 | 0.6 | 4.5 | 1.4 |
| 2 | 1 | 17 July 2009 | 1 | 11.2 | 3.4 | 6.5 | 3.2 | 10.5 | 4.9 | ||||
| 3 | 1 | 30 June 2010 | 1 | 6.8 | 95.6 | 14.3 | 1.4 | 3.1 | 1.5 | 9.8 | 3.7 | ||
| 4 | 2 | 17 July 2009 | 1 | 69°03′14.3″ | 20°52′07.3″ | 100.1 | 7.9 | 8.6 | 4.7 | 2.0 | 12.2 | 7.7 | |
| 5 | 2 | 05 July 2010 | 1 | 7.2 | 73.3 | 5.4 | 0.7 | 1.3 | 0.7 | 7.7 | 3.9 | ||
| 8 | 3 | 30 June 2010 | 0.5 | 69°02′49.2″ | 20°51′38.2″ | 6.4 | 30.6 | 0.9 | 0.7 | 1.7 | 0.8 | 2.0 | 1.3 |
| 6 | 3 | 17 July 2009 | 1 | 167.2 | 2.0 | 3.1 | 8.6 | 1.9 | 19.1 | 17.9 | |||
| 7 | 3 | 30 June 2010 | 1 | 6.3 | 47.1 | 2.4 | 0.7 | 2.8 | 1.2 | 3.4 | 2.3 | ||
| 9 | 8 | 17 July 2009 | 1 | 69°02′59.5″ | 20°51′51.8″ | 75.1 | 3.6 | 1.8 | 2.9 | 0.5 | 7.5 | 5.6 | |
| 10 | 8 | 05 July 2010 | 1 | 6.4 | 68.9 | 2.4 | 1.0 | 1.9 | 0.3 | 5.8 | 4.2 | ||
| 11 | 8 | 17 July 2009 | 2 | 128.4 | 54.3 | 1.4 | 4.2 | 4.1 | 12.9 | 12.7 | |||
| 12 | 8 | 05 July 2010 | 2 | 6.3 | 131.7 | 48.6 | 1.0 | 2.7 | 1.6 | 11.2 | 8.7 | ||
| 13 | 9 | 17 July 2009 | 1 | 69°03′09.7″ | 20°51′31.4″ | 230.0 | 112.1 | 3.2 | 6.2 | 3.8 | 25.4 | 26.8 | |
| 14 | 9 | 05 July 2010 | 1 | 7.4 | 170.2 | 69.7 | 1.0 | 2.5 | 1.3 | 14.8 | 13.5 | ||
| No. | Sampling Point | Date | Coordinates | pH | El.cond. | SO42− | Cl− | Na+ | K+ | Ca2+ | Mg2+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N°) | (E°) | μS·cm−1 | mg·L−1 | |||||||||
| 1 | 1 | 11 August 2008 | 69°03′16.8″ | 20°51′43.8″ | 6.4 | 29.6 | 0.7 | 1.1 | 0.3 | 0.1 | 0.1 | 0.1 |
| 2 | 1 | 17 July 2009 | 7.6 | 44.8 | 6.5 | 1.0 | 1.9 | 0.4 | 9.7 | 1.2 | ||
| 3 | 1 | 05 July 2010 | 7.5 | 36.5 | 5.3 | 0.9 | 1.8 | 0.3 | 6.7 | 0.7 | ||
| 4 | 3 | 17 July 2009 | 69°02′49.2″ | 20°51′38.2″ | 37.5 | 3.3 | 1.2 | 2.2 | 0.4 | 7.1 | 1.4 | |
| 5 | 3 | 05 July 2010 | 7.2 | 25.1 | 2.2 | 0.7 | 1.4 | 0.5 | 3.4 | 0.7 | ||
| 6 | 5 | 26 July 2017 | 69°02′35.9″ | 20°52′11.8″ | 7.9 | 98.3 | 21.0 | 0.1 | 1.4 | 1.3 | 10.0 | 3.8 |
| 7 | 6 | 26 July 2017 | 69°02′45.9″ | 20°52′05.3″ | 7.6 | 123.9 | 34.0 | 2.3 | 2.2 | 1.6 | 13.0 | 4.2 |
| 8 | 7 | 26 July 2017 | 69°02′47.8″ | 20°51′46.3″ | 8.2 | 211.1 | 63.0 | 1.9 | 2.0 | 0.8 | 23.1 | 8.1 |
| 9 | 8 | 17 July 2009 | 69°02′59.5″ | 20°51′51.8″ | 8.1 | 125.6 | 61.6 | 1.4 | 2.4 | 1.3 | 25.4 | 8.7 |
| 10 | 8 | 05 July 2010 | 7.1 | 116.2 | 41.9 | 0.8 | 1.8 | 0.8 | 17.5 | 4.9 | ||
| 11 | 9 | 17 July 2009 | 69°03′09.7″ | 20°51′31.4″ | 7.3 | 245.0 | 159.8 | 1.6 | 3.2 | 1.5 | 47.2 | 19.9 |
| 12 | 9 | 30 June 2010 | 7.4 | 172.6 | 73.0 | 1.1 | 2.3 | 1.0 | 24.7 | 0.1 | ||
| 13 | 10 | 12 August 2008 | 69°02′58.0″ | 20°49′39.3″ | 5.7 | 13.0 | 0.8 | 0.5 | 0.2 | 0.1 | 0.2 | 0.1 |
| 14 | 10 | 07 July 2009 | 6.8 | 12.7 | 1.4 | 1.1 | 1.3 | 0.4 | 2.5 | 0.2 | ||
| 15 | 10 | 08 July 2010 | 7.0 | 7.0 | 0.9 | 0.4 | 0.5 | 0.1 | 1.4 | 0.1 | ||
| 16 | 11 | 18 May 2008 | 69°05′14.9″ | 20°45′33.4″ | 7.2 | 22.8 | 1.4 | 2.1 | 1.5 | 0.4 | 1.8 | 0.4 |
| 17 | 12 | 27 May 2008 | 69°05′08.6″ | 20°47′20.5″ | 7.3 | 16.7 | 1.0 | 1.5 | 1.0 | 0.5 | 1.6 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raidla, V.; Kaup, E.; Hade, S.; Ivask, J.; Soesoo, A. Geochemical Processes Controlling Ionic Composition of Water in the Catchments of Lakes Saana and Saanalampi in the Kilpisjärvi Area of North Scandinavia. Geosciences 2019, 9, 174. https://doi.org/10.3390/geosciences9040174
Raidla V, Kaup E, Hade S, Ivask J, Soesoo A. Geochemical Processes Controlling Ionic Composition of Water in the Catchments of Lakes Saana and Saanalampi in the Kilpisjärvi Area of North Scandinavia. Geosciences. 2019; 9(4):174. https://doi.org/10.3390/geosciences9040174
Chicago/Turabian StyleRaidla, Valle, Enn Kaup, Sigrid Hade, Jüri Ivask, and Alvar Soesoo. 2019. "Geochemical Processes Controlling Ionic Composition of Water in the Catchments of Lakes Saana and Saanalampi in the Kilpisjärvi Area of North Scandinavia" Geosciences 9, no. 4: 174. https://doi.org/10.3390/geosciences9040174
APA StyleRaidla, V., Kaup, E., Hade, S., Ivask, J., & Soesoo, A. (2019). Geochemical Processes Controlling Ionic Composition of Water in the Catchments of Lakes Saana and Saanalampi in the Kilpisjärvi Area of North Scandinavia. Geosciences, 9(4), 174. https://doi.org/10.3390/geosciences9040174