Re-Evaluating the Age of Deep Biosphere Fossils in the Lockne Impact Structure
Abstract
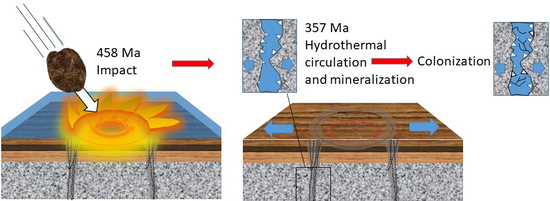
:1. Introduction
2. Geological Setting
3. Sample Material
4. Methods
4.1. Secondary Ion Mass Spectrometry (SIMS) Stable Isotope Analysis
4.2. Laser Ablation Multi-Collector Inductively Coupled Plasma Mass Spectrometry (LA-MC-ICP-MS) 87Sr/86Sr
4.3. LA-ICP-MS Analyses for Rb-Sr Dating
5. Results
5.1. Isotope Compositions
5.2. Rb/Sr Dating
6. Discussion
6.1. Ancient Methanogenesis in the Fracture Voids
6.2. New Age Constraints for the Fracture Assemblage in the Impact Structure and Microbial Communities
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Versh, E.; Kirsimäe, K.; Jõeleht, A. Development of potential ecological niches in impact-induced hydrothermal systems: The small-to-medium size impacts. Planet. Space Sci. 2006, 54, 1567–1574. [Google Scholar] [CrossRef]
- Osinski, G.R.; Tornabene, L.L.; Banerjee, N.R.; Cockell, C.S.; Flemming, R.; Izawa, M.R.; McCutcheon, J.; Parnell, J.; Preston, L.J.; Pickersgill, A.E.; et al. Impact-generated hydrothermal systems on Earth and Mars. Icarus 2013, 224, 347–363. [Google Scholar] [CrossRef]
- Kring, D.A. Impact events and their effect on the origin, evolution, and distribution of life. GSA today 2000, 10, 1–7. [Google Scholar]
- Cockell, C.S. The origin and emergence of life under impact bombardment. Philos. Trans. Soc. B: Boil. Sci. 2006, 361, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Lee, P. The biology of impact craters – a review. Boil. Rev. 2002, 77, 279–310. [Google Scholar]
- Naumov, M.V. Principal features of impact-generated hydrothermal circulation systems: Mineralogical and geochemical evidence. Geofluids 2002, 5, 165–184. [Google Scholar] [CrossRef]
- Ames, D.E.; Watkinson, D.H.; Parrish, R.R. Dating of a regional hydrothermal system induced by the 1850 Ma Sudbury impact event. Geology 1998, 26, 447. [Google Scholar] [CrossRef]
- Jõeleht, A.; Kirsimäe, K.; Plado, J.; Versh, E.; Ivanov, B. Cooling of the Kärdla impact crater: II. Impact and geothermal modeling. Meteor. Planet. Sci. 2005, 40, 21–33. [Google Scholar] [CrossRef]
- Arp, G.; Kolepka, C.; Simon, K.; Karius, V.; Nolte, N.; Hansen, B.T. New evidence for persistent impact-generated hydrothermal activity in the Miocene Ries impact structure, Germany. Meteorit. Planet. Sci. 2013, 48, 2491–2516. [Google Scholar] [CrossRef]
- Schmieder, M.; Jourdan, F. The Lappajärvi impact structure (Finland): Age, duration of crater cooling, and implications for early life. Geochim. Cosmochim. Acta 2013, 112, 321–339. [Google Scholar] [CrossRef]
- Kenny, G.G.; Schmieder, M.; Whitehouse, M.J.; Nemchin, A.A.; Morales, L.F.; Buchner, E.; Bellucci, J.J.; Snape, J.F. A new U-Pb age for shock-recrystallised zircon from the Lappajärvi impact crater, Finland, and implications for the accurate dating of impact events. Geochim. Cosmochim. Acta 2019, 245, 479–494. [Google Scholar] [CrossRef]
- Parnell, J.; Boyce, A.; Thackrey, S.; Muirhead, D.; Lindgren, P.; Mason, C.; Taylor, C.; Still, J.; Bowden, S.; Osinski, G.R.; et al. Sulfur isotope signatures for rapid colonization of an impact crater by thermophilic microbes. Geology 2010, 38, 271–274. [Google Scholar] [CrossRef]
- Sapers, H.M.; Osinski, G.R.; Banerjee, N.R.; Preston, L.J. Enigmatic tubular features in impact glass. Geology 2014, 42, 471–474. [Google Scholar] [CrossRef]
- Lindgren, P.; Ivarsson, M.; Neubeck, A.; Broman, C.; Henkel, H.; Holm, N.G. Putative fossil life in a hydrothermal system of the Dellen impact structure, Sweden. Int. J. Astrobiol. 2010, 9, 137–146. [Google Scholar] [CrossRef]
- Hode, T.; Cady, S.L.; von Dalwigk, I.; Kristiansson, P. Evidence of Ancient Microbial Life in an Impact Structure and Its Implications for Astrobiology. In From Fossils to Astrobiology: Records of Life on Earth and Search for Extraterrestrial Biosignatures; Seckbach, J., Walsh, M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 249–273. [Google Scholar]
- Ivarsson, M.; Broman, C.; Sturkell, E.; Ormö, J.; Siljeström, S.; Van Zuilen, M.; Bengtson, S. Fungal colonization of an Ordovician impact-induced hydrothermal system. Sci. Rep. 2013, 3, 3487. [Google Scholar] [CrossRef]
- Ivarsson, M.; Holm, N.G.; Neubeck, A. The Deep Biosphere of the Subseafloor Igneous Crust. In Trace Metal Biogeochemistry and Ecology of Deep-Sea Hydrothermal Vent Systems; Demina, L.L., Galkin, V.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 143–166. [Google Scholar]
- Ivarsson, M.; Bengtson, S.; Skogby, H.; Lazor, P.; Broman, C.; Belivanova, V.; Marone, F. A Fungal-Prokaryotic Consortium at the Basalt-Zeolite Interface in Subseafloor Igneous Crust. PLOS ONE 2015, 10, e0140106. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.; Heim, C.; Roberts, N.M.; Zack, T.; Tillberg, M.; Broman, C.; Ivarsson, M.; Whitehouse, M.J.; Åström, M.E. Isotopic evidence for microbial production and consumption of methane in the upper continental crust throughout the Phanerozoic eon. Earth Planet. Sci. Lett. 2017, 470, 108–118. [Google Scholar] [CrossRef]
- Drake, H.; Whitehouse, M.J.; Heim, C.; Reiners, P.W.; Tillberg, M.; Hogmalm, K.J.; Dopson, M.; Broman, C.; Åström, M.E. Unprecedented 34 S-enrichment of pyrite formed following microbial sulfate reduction in fractured crystalline rocks. Geobiology 2018, 16, 556–574. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.; Ivarsson, M.; Tillberg, M.; Whitehouse, M.J.; Kooijman, E. Ancient Microbial Activity in Deep Hydraulically Conductive Fracture Zones within the Forsmark Target Area for Geological Nuclear Waste Disposal, Sweden. Geosciences 2018, 8, 211. [Google Scholar] [CrossRef]
- Tillberg, M.; Drake, H.; Zack, T.; Hogmalm, J.; Åström, M. In Situ Rb-Sr Dating of Fine-grained Vein Mineralizations Using LA-ICP-MS. Procedia Earth Planet. Sci. 2017, 17, 464–467. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef]
- Drake, H.; Åström, M.E.; Heim, C.; Broman, C.; Åström, J.; Whitehouse, M.; Ivarsson, M.; Siljestrom, S.; Sjövall, P. Extreme 13C depletion of carbonates formed during oxidation of biogenic methane in fractured granite. Nat. Commun. 2015, 6, 7020. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L.; Whitehouse, M.; Sandberg, B.; Blomfeldt, T.; Åström, M.E. Extreme fractionation and micro-scale variation of sulphur isotopes during bacterial sulphate reduction in deep groundwater systems. Geochim. Cosmochim. Acta 2015, 161, 1–18. [Google Scholar] [CrossRef]
- Drake, H.; Åström, M.E.; Tullborg, E.-L.; Whitehouse, M.; Fallick, A.E. Variability of sulphur isotope ratios in pyrite and dissolved sulphate in granitoid fractures down to 1km depth – Evidence for widespread activity of sulphur reducing bacteria. Geochim. Cosmochim. Acta 2013, 102, 143–161. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M. The role of anaerobic fungi in fundamental biogeochemical cycles in the deep biosphere. Fungal Boil. Rev. 2018, 32, 20–25. [Google Scholar] [CrossRef]
- Alwmark, C.; Schmitz, B. Extraterrestrial chromite in the resurge deposits of the early Late Ordovician Lockne crater, central Sweden. Earth Planet. Sci. Lett. 2007, 253, 291–303. [Google Scholar] [CrossRef]
- Shuvalov, V.; Ormö, J.; Lindström, M. Hydrocode Simulation of the Lockne Marine Target Impact Event. In Impact Tectonics; Koeberl, C., Henkel, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 405–422. [Google Scholar]
- Högdahl, K. 1.86–1.85 Ga intrusive ages of K-feldspar megacryst-bearing granites in the type area of the Revsund granites in Jämtland County, central Sweden. GFF 2000, 122, 359–366. [Google Scholar] [CrossRef]
- Lindström, M.; Lundqvist, J.; Lundqvist, T. Sveriges Geologi Från Urtid till Nutid; Studentlitteratur: Lund, Sweden, 2000. [Google Scholar]
- Lindström, M.; Sturkell, E.F. Geology of the Early Palaeozoic Lockne impact structure, Central Sweden. Tectonophysics 1992, 216, 169–185. [Google Scholar] [CrossRef]
- Sturkell, E.F.; Broman, C.; Forsberg, P.; Torssander, P. Impact-related hydrothermal activity in the Lockne impact structure, Jämtland, Sweden. Eur. J. Miner. 1998, 10, 589–606. [Google Scholar] [CrossRef]
- Crowe, D.E.; Vaughan, R.G. Characterization and use of isotopically homogeneous standards for in situ laser microprobe analysis of 34 S/ 32 S ratios. Am. Miner. 1996, 81, 187–193. [Google Scholar] [CrossRef]
- Kiel, S.; Glodny, J.; Birgel, D.; Bulot, L.G.; Campbell, K.A.; Gaillard, C.; Graziano, R.; Kaim, A.; Lazăr, I.; Sandy, M.R.; et al. The Paleoecology, Habitats, and Stratigraphic Range of the Enigmatic Cretaceous Brachiopod Peregrinella. PLOS ONE 2014, 9, e109260. [Google Scholar] [CrossRef] [PubMed]
- Mokadem, F.; Parkinson, I.J.; Hathorne, E.C.; Anand, P.; Allen, J.T.; Burton, K.W. High-precision radiogenic strontium isotope measurements of the modern and glacial ocean: Limits on glacial–interglacial variations in continental weathering. Earth Planet. Sci. Lett. 2015, 415, 111–120. [Google Scholar] [CrossRef]
- Zack, T.; Hogmalm, K.J. Laser ablation Rb/Sr dating by online chemical separation of Rb and Sr in an oxygen-filled reaction cell. Chem. Geol. 2016, 437, 120–133. [Google Scholar] [CrossRef]
- Hogmalm, K.J.; Zack, T.; Karlsson, A.K.-O.; Sjöqvist, A.S.L.; Garbe-Schönberg, D. In situ Rb–Sr and K–Ca dating by LA-ICP-MS/MS: an evaluation of N 2 O and SF 6 as reaction gases. J. Anal. At. Spectrom. 2017, 32, 305–313. [Google Scholar] [CrossRef]
- Nebel, O.; Mezger, K.; Scherer, E.; Münker, C. High precision determinations of 87Rb/85Rb in geologic materials by MC-ICP-MS. Int. J. Mass Spectrom. 2005, 246, 10–18. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610-617 Glasses Following ISO Guidelines. Geostand. Geoanalytical 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Elburg, M.; Vroon, P.; Van Der Wagt, B.; Tchalikian, A. Sr and Pb isotopic composition of five USGS glasses (BHVO-2G, BIR-1G, BCR-2G, TB-1G, NKT-1G). Chem. Geol. 2005, 223, 196–207. [Google Scholar] [CrossRef]
- Villa, I.; De Bièvre, P.; Holden, N.; Renne, P. IUPAC-IUGS recommendation on the half life of 87 Rb. Geochim. Cosmochim. Acta 2015, 164, 382–385. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P.; Whitehouse, M. Biogenic processes in crystalline bedrock fractures indicated by carbon isotope signatures of secondary calcite. Appl. Geochem. 2016, 67, 30–41. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.; Pitkanen, P.; Whitehouse, M. Implications of sulfur isotope fractionation in fracture-filling sulfides in crystalline bedrock, Olkiluoto, Finland. Appl. Geochem. 2013, 32, 52–69. [Google Scholar] [CrossRef]
- Knittel, K.; Boetius, A. Anaerobic Oxidation of Methane: Progress with an Unknown Process. Annu. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Peckmann, J.; Thiel, V. Carbon cycling at ancient methane–seeps. Chem. Geol. 2004, 205, 443–467. [Google Scholar] [CrossRef]
- Canfield, D.E.; Olesen, C.A.; Cox, R.P. Temperature and its control of isotope fractionation by a sulphate-reducing bacterium. Geochim. Cosmochim. Acta 2006, 70, 548–561. [Google Scholar] [CrossRef]
- Johnston, D.T.; Wing, B.A.; Farquhar, J.; Kaufman, A.J.; Strauss, H.; Lyons, T.W.; Kah, L.C.; Canfield, D.E. Active Microbial Sulfur Disproportionation in the Mesoproterozoic. Science 2005, 310, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Boehme, S.E.; Blair, N.E.; Chanton, J.P.; Martens, C.S. A mass balance of 13C and 12C in an organic-rich methane-producing marine sediment. Geochim. Cosmochim. Acta 1996, 60, 3835–3848. [Google Scholar] [CrossRef]
- Budai, J.M.; Walter, L.M.; Ku, T.C.W.; Martini, A.M. Fracture-fill calcite as a record of microbial methanogenesis and fluid migration: a case study from the Devonian Antrim Shale, Michigan Basin. Geofluids 2002, 2, 163–183. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L.; Hogmalm, K.J.; Åström, M.E. Trace metal distribution and isotope variations in low-temperature calcite and groundwater in granitoid fractures down to 1km depth. Geochim. Cosmochim. Acta 2012, 84, 217–238. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L. Episodic fluid migration in the Fennoscandian Shield recorded by stable isotopes, rare earth elements and fluid inclusions in fracture minerals at Forsmark, Sweden. Chem. Geol. 2009, 266, 126–142. [Google Scholar] [CrossRef]
- Kietäväinen, R.; Purkamo, L. The origin, source, and cycling of methane in deep crystalline rock biosphere. Front. Microbiol. 2015, 6, 725. [Google Scholar] [CrossRef]
- Etiope, G.; Lollar, B.S. ABIOTIC METHANE ON EARTH. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Pallasser, R. Recognising biodegradation in gas/oil accumulations through the δ 13 C compositions of gas components. Org. Geochem. 2000, 31, 1363–1373. [Google Scholar] [CrossRef]
- Sim, M.S.; Bosak, T.; Ono, S. Large sulfur isotope fractionation does not require disproportionation. Science 2011, 333, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Machel, H.G.; Krouse, H.R.; Sassen, R. Products and distinguishing criteria of bacterial and thermochemical sulfate reduction. Appl. Geochem. 1995, 10, 373–389. [Google Scholar] [CrossRef]
- Strauss, H. The isotopic composition of sedimentary sulfur through time. Palaeogeogr. Palaeoclim. Palaeoecol. 1997, 132, 97–118. [Google Scholar] [CrossRef]
- Field, C.W.; Fifarek, R.H. Light stable-isotopes systematics in the epithermal environment. Rev. Econ. Geology 1985, 2, 99–128. [Google Scholar]
- Kiyosu, Y.; Krouse, H.R. The role of organic acid in the abiogenic reduction of sulfate and the sulfur isotope effect. Geochem. J. 1990, 24, 21–27. [Google Scholar] [CrossRef]
- Vlierboom, F.; Collini, B.; Zumberge, J. The occurrence of petroleum in sedimentary rocks of the meteor impact crater at Lake Siljan, Sweden. Org. Geochem. 1986, 10, 153–161. [Google Scholar] [CrossRef]
- Roberts, H.H.; Aharon, P. Hydrocarbon-derived carbonate buildups of the northern Gulf of Mexico continental slope: A review of submersible investigations. Geo-Marine Lett. 1994, 14, 135–148. [Google Scholar] [CrossRef]
- Koistinen, T.; Stephens, M.B.; Bogatchev, V.; Nordgulen, Ø.; Wennerström, M.; Korkonen, J. Geological Map of the Fennoscandian Shield Scale 1:2,000,000; Geological Surveys of Finland, Norway and Sweden and the Northwest Department of Natural Resources of Russia: Espoo, Finland, 2001. [Google Scholar]
- Guenthner, W.R.; Reiners, P.W.; Drake, H.; Tillberg, M. Zircon, titanite, and apatite (U-Th)/He ages and age-eU correlations from the Fennoscandian Shield, southern Sweden. Tectonics 2017, 36, 1254–1274. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L.; De Torres, T.; Ortiz, J.E. The occurrence and potential origin of asphaltite in bedrock fractures, Forsmark, central Sweden. GFF 2006, 128, 233–242. [Google Scholar] [CrossRef]
- Parnell, J.; Baba, M.; Bowden, S.; Muirhead, D. Subsurface biodegradation of crude oil in a fractured basement reservoir, Shropshire, UK. J. Geol. Soc. 2017, 174, 655–666. [Google Scholar] [CrossRef]
- McCready, A.J.; Stumpfl, E.F.; Melcher, F. U/Th-rich bitumen in Archean granites and Palaeoproterozoic metasediments, Rum Jungle Mineral Field, Australia: implications for mineralizing fluids. Geofluids 2003, 3, 147–159. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L. Paleohydrogeological events recorded by stable isotopes, fluid inclusions and trace elements in fracture minerals in crystalline rock, Simpevarp area, SE Sweden. Appl. Geochem. 2009, 24, 715–732. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P. Indications for the past redox environments in deep groundwaters from the isotopic composition of carbon and oxygen in fracture calcite, Olkiluoto, SW Finland. Isot. Environ. Heal. Stud. 2010, 46, 370–391. [Google Scholar] [CrossRef]
- Alm, E.; Sundblad, K.; Huhma, H. Sm-Nd Isotope Determinations of Low-Temperature Fluorite-Calcite-Galena Mineralization in the Margins of the Fennoscandian Shield; Report R-05-66; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2005; p. 58. [Google Scholar]
- Broman, C.; Sturkell, E.; Fallick, A.E. Oxygen isotopes and implications for the cavity-grown quartz crystals in the Lockne impact structure, Sweden. GFF 2011, 133, 101–107. [Google Scholar] [CrossRef]
- Maskenskaya, O.M.; Drake, H.; Broman, C.; Hogmalm, J.K.; Czuppon, G.; Astrom, M.E. Source and character of syntaxial hydrothermal calcite veins in Paleoproterozoic crystalline rocks revealed by fine-scale investigations. Geofluids 2014, 14, 495–511. [Google Scholar] [CrossRef]
- Simpson, S.; Boyce, A.; Lambert, P.; Lindgren, P.; Lee, M.; Simpson, S.; Boyce, A. Evidence for an impact-induced biosphere from the δ 34 S signature of sulphides in the Rochechouart impact structure, France. Earth Planet. Sci. Lett. 2017, 460, 192–200. [Google Scholar] [CrossRef]
- Bottomley, D.; Katz, A.; Chan, L.; Starinsky, A.; Douglas, M.; Clark, I.; Raven, K. The origin and evolution of Canadian Shield brines: evaporation or freezing of seawater? New lithium isotope and geochemical evidence from the Slave craton. Chem. Geol. 1999, 155, 295–320. [Google Scholar] [CrossRef]
- Lüders, V.; Rickers, K. Fluid inclusion evidence for impact-related hydrothermal fluid and hydrocarbon migration in Creataceous sediments of the ICDP-Chicxulub drill core Yax-1. Meteorit. Planet. Sci. 2004, 39, 1187–1197. [Google Scholar] [CrossRef]
- Zurcher, L.; Kring, D.A.; Barton, M.D.; Dettman, D.; Rollog, M. Stable isotope record of post-impact fluid activity in the Yaxcopoil-1 borehole, Chicxulub impact structure, Mexico T. In Large Meteorite Impacts III; Kenkmann, T., Hörz, F., Deutsch, A., Eds.; Geological Society of America: Boulder, CO, USA, 2005; pp. 223–238. [Google Scholar]
- Katz, A.; Starinsky, A.; Marion, G.M. Saline waters in basement rocks of the Kaapvaal Craton, South Africa. Chem. Geol. 2011, 289, 163–170. [Google Scholar] [CrossRef]
- Gascoyne, M. Hydrogeochemistry, groundwater ages and sources of salts in a granitic batholith on the Canadian Shield, southeastern Manitoba. Appl. Geochem. 2004, 19, 519–560. [Google Scholar] [CrossRef]
- Ivarsson, M.; Bengtson, S.; Drake, H.; Francis, W. Fungi in Deep Subsurface Environments. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Ladenberger, A.; Be’eri-Shlevin, Y.; Claesson, S.; Gee, D.G.; Majka, J.; Romanova, I.V. Tectonometamorphic evolution of the Åreskutan Nappe–Caledonian history revealed by SIMS U–Pb zircon geochronology. Geol. Soc. London Publ. 2014, 390, 337–368. [Google Scholar] [CrossRef]
- Grier, J.A.; Swindle, T.D.; Kring, D.A.; Melosh, H.J. Argon-40/argon-39 analyses of samples from the Gardnos impact structure, Norway. Meteorit. Planet. Sci. 1999, 34, 803–807. [Google Scholar] [CrossRef]
- Schmieder, M.; Tohver, E.; Jourdan, F.; Denyszyn, S.; Haines, P. Zircons from the Acraman impact melt rock (South Australia): Shock metamorphism, U–Pb and 40 Ar/ 39 Ar systematics, and implications for the isotopic dating of impact events. Geochim. Cosmochim. Acta 2015, 161, 71–100. [Google Scholar] [CrossRef]
- Buchner, E.; Schmieder, M.; Schwarz, W.H.; Trieloff, M.; Hopp, J.; Spray, J.G. Dating the Charlevoix Impact Structure (Québec, Canada) – A Tough Nut to Crack in 40Ar/39Ar Geochronology. In Proceedings of the 41st Lunar and Planetary Science Conference, The Woodlands, TX, USA, 1–5 March 2010; p. 41. [Google Scholar]
- Ksienzyk, A.K.; Wemmer, K.; Jacobs, J.; Fossen, H.; Schomberg, A.C.; Süssenberger, A.; Lünsdorf, N.K.; Bastesen, E. Post-Caledonian brittle deformation in the Bergen area, West Norway: Results from K–Ar illite fault gouge dating. Nor. J. Geol. 2016, 96, 275–299. [Google Scholar] [CrossRef]
- Steltenpohl, M.G.; Carter, B.T.; Andresen, A.; Zeltner, D.L. 40Ar/39Ar Thermochronology of Late- and Postorogenic Extension in the Caledonides of North-Central Norway. J. Geol. 2009, 117, 399–414. [Google Scholar] [CrossRef]
- Hacker, B.R.; Gans, P.B. Continental collisions and the creation of ultrahigh-pressure terranes: Petrology and thermochronology of nappes in the central Scandinavian Caledonides. GSA Bull. 2005, 117, 117. [Google Scholar] [CrossRef]
- Eide, E.A.; Torsvik, T.H.; Andersen, T.B.; Arnaud, N.O. Early Carboniferous Unroofing in Western Norway: A Tale of Alkali Feldspar Thermochronology. J. Geol. 1999, 107, 353–374. [Google Scholar] [CrossRef]




| Crystal/Reference | 32S cps (× 109) | 34S/32S | ± abs | δ34SCDT (°) | ±σ (°) |
|---|---|---|---|---|---|
| Drift Corrected | |||||
| Reference | 0.66 | 0.0442764 | 0.0000017 | 0.36 | 0.06 |
| Reference | 0.68 | 0.0442713 | 0.0000023 | 0.24 | 0.07 |
| 1 | 0.66 | 0.0443742 | 0.0000021 | 2.57 | 0.07 |
| 1 | 0.66 | 0.0443723 | 0.0000018 | 2.52 | 0.07 |
| 1 | 0.65 | 0.0443817 | 0.0000030 | 2.73 | 0.09 |
| 2 | 0.66 | 0.0443697 | 0.0000026 | 2.46 | 0.08 |
| 2 | 0.66 | 0.0443756 | 0.0000018 | 2.60 | 0.07 |
| 3 | 0.65 | 0.0443733 | 0.0000019 | 2.55 | 0.07 |
| Reference | 0.66 | 0.0442731 | 0.0000013 | 0.28 | 0.06 |
| 3 | 0.66 | 0.0443553 | 0.0000031 | 2.14 | 0.09 |
| 3 | 0.66 | 0.0443692 | 0.0000023 | 2.45 | 0.07 |
| 3 | 0.66 | 0.0443696 | 0.0000020 | 2.46 | 0.07 |
| 3 | 0.66 | 0.0443794 | 0.0000029 | 2.68 | 0.08 |
| 4 | 0.66 | 0.0443826 | 0.0000045 | 2.76 | 0.11 |
| 4 | 0.66 | 0.0443832 | 0.0000020 | 2.77 | 0.07 |
| Reference | 0.66 | 0.0442755 | 0.0000018 | 0.34 | 0.07 |
| 4 | 0.66 | 0.0443779 | 0.0000025 | 2.65 | 0.08 |
| 5 | 0.65 | 0.0443602 | 0.0000021 | 2.25 | 0.07 |
| 6 | 0.65 | 0.0443216 | 0.0000023 | 1.38 | 0.07 |
| 7 | 0.66 | 0.0443781 | 0.0000026 | 2.65 | 0.08 |
| 8 | 0.66 | 0.0443895 | 0.0000026 | 2.91 | 0.08 |
| 8 | 0.66 | 0.0443752 | 0.0000024 | 2.59 | 0.07 |
| Reference | 0.66 | 0.0442745 | 0.0000027 | 0.31 | 0.08 |
| 9 | 0.65 | 0.0443958 | 0.0000019 | 3.05 | 0.07 |
| 9 | 0.65 | 0.0444002 | 0.0000023 | 3.15 | 0.07 |
| 9 | 0.66 | 0.0443685 | 0.0000030 | 2.44 | 0.08 |
| 9 | 0.66 | 0.0443857 | 0.0000017 | 2.83 | 0.06 |
| 10 | 0.66 | 0.0444065 | 0.0000030 | 3.30 | 0.09 |
| 10 | 0.65 | 0.0444176 | 0.0000021 | 3.55 | 0.07 |
| Reference | 0.64 | 0.0442718 | 0.0000022 | 0.25 | 0.07 |
| 10 | 0.64 | 0.0444125 | 0.0000028 | 3.43 | 0.08 |
| 10 | 0.63 | 0.0444023 | 0.0000035 | 3.20 | 0.09 |
| 11 | 0.63 | 0.0443028 | 0.0000034 | 0.95 | 0.09 |
| 11 | 0.64 | 0.0442781 | 0.0000025 | 0.39 | 0.08 |
| 11 | 0.64 | 0.0444033 | 0.0000022 | 3.22 | 0.07 |
| 12 | 0.64 | 0.0443509 | 0.0000027 | 2.04 | 0.08 |
| Reference | 0.64 | 0.0442744 | 0.0000020 | 0.31 | 0.07 |
| 13 | 0.63 | 0.0443912 | 0.0000024 | 2.95 | 0.07 |
| 13 | 0.64 | 0.0444193 | 0.0000022 | 3.59 | 0.07 |
| 14 | 0.63 | 0.0443902 | 0.0000027 | 2.93 | 0.08 |
| 14 | 0.64 | 0.0443839 | 0.0000021 | 2.79 | 0.07 |
| 14 | 0.63 | 0.0443679 | 0.0000025 | 2.42 | 0.08 |
| 14 | 0.66 | 0.0443547 | 0.0000032 | 2.12 | 0.09 |
| 15 | 0.64 | 0.0443929 | 0.0000042 | 2.99 | 0.11 |
| Reference | 0.65 | 0.0442740 | 0.0000026 | 0.30 | 0.08 |
| Reference | 0.65 | 0.0442776 | 0.0000023 | 0.38 | 0.07 |
| Reference | 0.65 | 0.0442705 | 0.0000024 | 0.22 | 0.07 |
| Crystal/Reference | 12C cps [× 109] | 13C/12C (Drift Corrected) | ±abs (°) | δ13CPDB (°) | ±σ (°) | Sr isotopes (Sampling Time) | 87Sr/86Sr 1 | 2SD 2 | 84Sr/86Sr | 2SE | 87Rb/86Sr3 | 2SE | Total Sr-Beam |
| Reference | 0.0179 | 0.0108741 | 0.0000025 | 0.20 | 0.45 | ||||||||
| Reference | 0.0181 | 0.0108747 | 0.0000030 | 0.26 | 0.48 | ||||||||
| Reference | 0.0180 | 0.0108753 | 0.0000025 | 0.31 | 0.45 | ||||||||
| Reference | 0.0180 | 0.0108720 | 0.0000025 | 0.01 | 0.45 | ||||||||
| 181 m, crystal 1 | 0.0184 | 0.0107828 | 0.0000030 | −8.20 | 0.47 | ||||||||
| 181 m, crystal 1 | 0.0187 | 0.0107763 | 0.0000027 | −8.79 | 0.46 | ||||||||
| 181 m, crystal 1 | 0.0184 | 0.0107798 | 0.0000032 | −8.47 | 0.48 | ||||||||
| Reference | 0.0180 | 0.0108795 | 0.0000025 | 0.70 | 0.45 | ||||||||
| 181 m, crystal 1 | 0.0184 | 0.0107996 | 0.0000025 | −6.65 | 0.45 | ||||||||
| 181 m, crystal 2 | 0.0184 | 0.0106878 | 0.0000030 | −16.93 | 0.47 | ||||||||
| 181 m, crystal 2 | 0.0186 | 0.0106803 | 0.0000025 | −17.62 | 0.44 | ||||||||
| 181 m, crystal 2 | 0.0176 | 0.0106614 | 0.0000032 | −19.37 | 0.48 | ||||||||
| 185 m, crystal 1 | 0.0185 | 0.0110004 | 0.0000025 | 11.82 | 0.45 | ||||||||
| 185 m, crystal 1 | 0.0184 | 0.0109904 | 0.0000041 | 10.90 | 0.54 | 31.0 | 0.7464 | 0.0013 | 0.0580 | 0.0038 | 0.00015 | 0.00047 | 0.167 |
| Reference | 0.0179 | 0.0108689 | 0.0000037 | −0.27 | 0.51 | ||||||||
| 185 m, crystal 1 | 0.0186 | 0.0110087 | 0.0000028 | 12.58 | 0.47 | 30.6 | 0.7466 | 0.0012 | 0.0602 | 0.0047 | <DL | 0.140 | |
| 185 m, crystal 1 | 0.0184 | 0.0109799 | 0.0000042 | 9.93 | 0.55 | 31.0 | 0.7471 | 0.0012 | 0.0548 | 0.0035 | 0.00014 | 0.00028 | 0.238 |
| 185 m, crystal 1 | 0.0183 | 0.0110098 | 0.0000034 | 12.68 | 0.50 | 31.0 | 0.7444 | 0.0015 | 0.0578 | 0.0047 | 0.00010 | 0.00051 | 0.144 |
| 185 m, crystal 1 | 0.0187 | 0.0109870 | 0.0000027 | 10.58 | 0.46 | ||||||||
| Reference | 0.0179 | 0.0108738 | 0.0000028 | 0.17 | 0.47 | ||||||||
| Reference | 0.0179 | 0.0108801 | 0.0000029 | 0.75 | 0.47 | ||||||||
| Reference | 0.0179 | 0.0108717 | 0.0000025 | −0.02 | 0.45 | ||||||||
| Reference | 0.0176 | 0.0108796 | 0.0000031 | 0.71 | 0.48 | ||||||||
| Reference | 0.0177 | 0.0108739 | 0.0000028 | 0.19 | 0.46 | ||||||||
| Reference | 0.0174 | 0.0108713 | 0.0000026 | −0.05 | 0.45 | ||||||||
| Measurements of Sr isotope reference materials Primary reference material: ’Ecnomiosa gerda’ | |||||||||||||
| Spot Number | Sampling Time | 87Sr/86Sr1 | 2SD2 | 84Sr/86Sr | 2SE | 87Rb/86Sr3 | 2SE | Total Sr-Beam | |||||
| 1 | 30.8 | 0.70914 | 0.00019 | 0.05629 | 0.00017 | 0.000055 | 0.000025 | 3.26 | |||||
| 2 | 30.4 | 0.70923 | 0.00015 | 0.05657 | 0.00018 | 0.000047 | 0.000017 | 3.77 | |||||
| 3 | 30.4 | 0.70906 | 0.00015 | 0.05646 | 0.00017 | 0.000007 | 0.000024 | 4.02 | |||||
| 4 | 30.8 | 0.70926 | 0.00016 | 0.05656 | 0.00014 | 0.000043 | 0.000016 | 4.36 | |||||
| 5 | 30.4 | 0.70914 | 0.00016 | 0.05646 | 0.00014 | 0.000055 | 0.000016 | 4.04 | |||||
| 6 | 30.8 | 0.70921 | 0.00015 | 0.05658 | 0.00017 | 0.000035 | 0.000016 | 4.10 | |||||
| 7 | 31.2 | 0.70913 | 0.00017 | 0.05656 | 0.00016 | 0.000031 | 0.000021 | 3.61 | |||||
| 8 | 30.4 | 0.70913 | 0.00019 | 0.05646 | 0.00013 | 0.000009 | 0.000015 | 4.22 | |||||
| 9 | 30.4 | 0.70915 | 0.00024 | 0.05642 | 0.00021 | 0.000015 | 0.000025 | 2.81 | |||||
| 10 | 30.4 | 0.70908 | 0.00021 | 0.05639 | 0.00020 | 0.000036 | 0.000021 | 3.29 | |||||
| 11 | 30.8 | 0.70923 | 0.00017 | 0.05650 | 0.00018 | 0.000043 | 0.000018 | 3.55 | |||||
| 12 | 30.4 | 0.70918 | 0.00018 | 0.05640 | 0.00019 | 0.000047 | 0.000019 | 3.51 | |||||
| 13 | 30.8 | 0.70923 | 0.00016 | 0.05643 | 0.00013 | 0.000065 | 0.000017 | 3.96 | |||||
| Average | 0.70917 | 0.05647 | |||||||||||
| 2 SD | 0.00012 | 0.00017 | |||||||||||
| Secondary reference material: modern oyster shell from Western Australia | |||||||||||||
| Spot Number | Sampling Time | 87Sr/86Sr1 | 2SD2 | 84Sr/86Sr | 2SE | 87Rb/86Sr3 | 2SE | Total Sr-Beam | |||||
| 1 | 29.2 | 0.70915 | 0.00019 | 0.05666 | 0.00032 | 0.000050 | 0.000036 | 2.23 | |||||
| 2 | 29.2 | 0.70932 | 0.00019 | 0.05664 | 0.00024 | 0.000079 | 0.000025 | 2.50 | |||||
| 3 | 29.6 | 0.70927 | 0.00020 | 0.05616 | 0.00026 | 0.000109 | 0.000030 | 2.50 | |||||
| Average | 0.70925 | 0.05649 | |||||||||||
| 2 SD | 0.00017 | 0.00057 | |||||||||||
| Primary 87Rb/86Sr Reference Material: BCR-2G | ||||||||||||||
| Spot No. | Spot Size (μm) | 87Rb/86Sr | 1s error (%) | 87Sr/86Sr | 1s Error (%) | ρ | Age Error | Ratio RSE | Signal Length (sec) | |||||
| 6 | 50 | 0.3917 | 0.75% | 0.70217 | 0.27% | 0.18 | - | 0.67% | 65 | |||||
| 25 | 50 | 0.3994 | 0.64% | 0.70428 | 0.32% | 0.23 | - | 0.81% | 65 | |||||
| 26 | 50 | 0.3966 | 0.62% | 0.70494 | 0.22% | 0.14 | - | 0.54% | 65 | |||||
| 47 | 50 | 0.3941 | 0.58% | 0.70713 | 0.30% | 0.20 | - | 0.74% | 65 | |||||
| 48 | 50 | 0.3865 | 0.67% | 0.70684 | 0.28% | 0.44 | - | 0.73% | 65 | |||||
| 72 | 50 | 0.3920 | 0.53% | 0.70481 | 0.28% | 0.28 | - | 0.68% | 65 | |||||
| 73 | 50 | 0.3841 | 0.61% | 0.70376 | 0.29% | 0.23 | - | 0.72% | 65 | |||||
| 95 | 50 | 0.3935 | 0.62% | 0.70451 | 0.27% | 0.17 | - | 0.64% | 65 | |||||
| 96 | 50 | 0.3895 | 0.53% | 0.70536 | 0.25% | 0.35 | - | 0.63% | 65 | |||||
| 119 | 50 | 0.3858 | 0.64% | 0.70474 | 0.22% | 0.32 | - | 0.59% | 65 | |||||
| 120 | 50 | 0.3914 | 0.70% | 0.70619 | 0.31% | 0.29 | - | 0.78% | 65 | |||||
| 146 | 50 | 0.3884 | 0.52% | 0.70788 | 0.25% | 0.16 | - | 0.61% | 65 | |||||
| 147 | 50 | 0.3933 | 0.59% | 0.70478 | 0.31% | 0.19 | - | 0.74% | 65 | |||||
| 170 | 50 | 0.3886 | 0.81% | 0.70568 | 0.38% | 0.39 | - | 1.00% | 50 | |||||
| 171 | 50 | 0.3870 | 0.57% | 0.70518 | 0.28% | 0.17 | - | 0.66% | 65 | |||||
| 191 | 50 | 0.3906 | 0.60% | 0.70494 | 0.27% | 0.28 | - | 0.70% | 65 | |||||
| 192 | 50 | 0.3896 | 0.60% | 0.70518 | 0.26% | 0.23 | - | 0.66% | 65 | |||||
| 213 | 50 | 0.3860 | 0.67% | 0.70455 | 0.28% | 0.34 | - | 0.77% | 65 | |||||
| 214 | 50 | 0.3819 | 0.65% | 0.70204 | 0.33% | 0.44 | - | 0.85% | 65 | |||||
| Average | 0.39 | 0.70500 | ||||||||||||
| SD | 1.12% | 0.21% | ||||||||||||
| Primary 87Sr/86Sr Reference Material: NIST-SRM-610 | ||||||||||||||
| Spot No. | Spot Size (μm) | 87Rb/86Sr | 1s Error (%) | 87Sr/86Sr | 1s Error (%) | ρ | Age Error | Ratio RSE | Signal Length (sec) | |||||
| 1 | 50 | 2.3244 | 0.36% | 0.70436 | 0.28% | 0.41 | - | 0.81% | 65 | |||||
| 2 | 50 | 2.3246 | 0.44% | 0.70910 | 0.23% | 0.37 | - | 0.69% | 65 | |||||
| 3 | 50 | 2.3651 | 0.38% | 0.71060 | 0.28% | 0.32 | - | 0.82% | 65 | |||||
| 4 | 50 | 2.3469 | 0.42% | 0.71208 | 0.25% | 0.25 | - | 0.73% | 65 | |||||
| 23 | 50 | 2.3611 | 0.41% | 0.71341 | 0.27% | 0.24 | - | 0.75% | 65 | |||||
| 24 | 50 | 2.3586 | 0.41% | 0.71120 | 0.34% | 0.36 | - | 1.00% | 65 | |||||
| 45 | 50 | 2.3320 | 0.35% | 0.70624 | 0.26% | 0.20 | - | 0.75% | 65 | |||||
| 46 | 50 | 2.3416 | 0.42% | 0.70980 | 0.32% | 0.33 | - | 0.90% | 65 | |||||
| 70 | 50 | 2.3375 | 0.68% | 0.70592 | 0.33% | 0.45 | - | 0.95% | 65 | |||||
| 71 | 50 | 2.3407 | 0.57% | 0.71322 | 0.32% | 0.39 | - | 0.92% | 65 | |||||
| 93 | 50 | 2.3685 | 0.51% | 0.71297 | 0.37% | 0.31 | - | 1.10% | 65 | |||||
| 94 | 50 | 2.3635 | 0.63% | 0.70978 | 0.27% | 0.26 | - | 0.77% | 55 | |||||
| 117 | 50 | 2.3586 | 0.41% | 0.70905 | 0.27% | 0.32 | - | 0.81% | 65 | |||||
| 118 | 50 | 2.3488 | 0.45% | 0.71169 | 0.24% | 0.28 | - | 0.69% | 65 | |||||
| 144 | 50 | 2.3456 | 0.51% | 0.71069 | 0.32% | 0.29 | - | 0.92% | 65 | |||||
| 145 | 50 | 2.3474 | 0.48% | 0.71028 | 0.23% | 0.33 | - | 0.70% | 65 | |||||
| 168 | 50 | 2.3556 | 0.53% | 0.70719 | 0.27% | 0.28 | - | 0.79% | 65 | |||||
| 169 | 50 | 2.3328 | 0.38% | 0.71201 | 0.29% | 0.33 | - | 0.83% | 65 | |||||
| 189 | 50 | 2.3042 | 0.43% | 0.70903 | 0.25% | 0.43 | - | 0.74% | 65 | |||||
| 190 | 50 | 2.2924 | 0.40% | 0.70751 | 0.25% | 0.33 | - | 0.74% | 65 | |||||
| 211 | 50 | 2.2880 | 0.42% | 0.71029 | 0.23% | 0.19 | - | 0.64% | 65 | |||||
| 212 | 50 | 2.2683 | 0.55% | 0.70698 | 0.30% | 0.36 | - | 0.87% | 40 | |||||
| Average | 2.3366 | 0.7097 | ||||||||||||
| SD | 1.16% | 0.35% | ||||||||||||
| Secondary Reference Material: La Posta | ||||||||||||||
| Spot No. | Spot Size (μm) | Mineral | 87Rb/86Sr | 1s error (%) | 87Sr/86Sr | 1s error (%) | ρ | Age Error | Ratio RSE | Signal Length (sec) | Status | Note | ||
| 15 | 50 | Biotite | 849 | 4.32% | 1.83 | 4.26% | 0.94 | 3.63% | 13.15% | 55 | ||||
| 16 | 50 | Biotite | 1025 | 5.23% | 2.03 | 5.03% | 0.94 | 4.29% | 29.62% | 55 | ||||
| 51 | 50 | Biotite | 891 | 21.45% | 1.97 | 21.22% | 0.95 | 9.81% | 22.48% | 10 | rejected | short signal | ||
| 52 | 50 | Biotite | 250 | 5.01% | 1.03 | 4.06% | 0.67 | 12.02% | 12.49% | 30 | ||||
| 53 | 50 | Biotite | 261 | 4.93% | 1.06 | 4.12% | 0.76 | 12.45% | 15.29% | 30 | ||||
| 99 | 50 | Biotite | 35.2 | 10.92% | 0.764 | 1.37% | 0.78 | 50.36% | 3.86% | 65 | rejected | unstable ablation, high 87Rb/86Sr error | ||
| 100 | 50 | Biotite | 261 | 3.54% | 1.06 | 2.73% | 0.76 | 6.07% | 6.75% | 58 | ||||
| 123 | 50 | Biotite | 512 | 4.34% | 1.39 | 3.56% | 0.87 | 4.91% | 11.94% | 60 | ||||
| 124 | 50 | Biotite | 858 | 4.61% | 1.83 | 4.31% | 0.92 | 3.60% | 11.24% | 67 | ||||
| 150 | 50 | Biotite | 140 | 5.14% | 0.896 | 2.49% | 0.65 | 12.29% | 8.81% | 37 | ||||
| 151 | 50 | Biotite | 185 | 4.11% | 0.947 | 3.19% | 0.75 | 17.51% | 15.68% | 60 | ||||
| 203 | 50 | Biotite | 53.4 | 14.51% | 0.904 | 4.84% | 0.25 | 39.23% | 11.82% | 8 | rejected | short signal | ||
| 204 | 50 | Biotite | 19.1 | 24.36% | 0.792 | 3.31% | 0.13 | 59.06% | 8.89% | 15 | rejected | unstable ablation, high 87Rb/86Sr error | ||
| Sample: 216m | ||||||||||||
| Spot No. | Spot Size (μm) | Mineral | 87Rb/86Sr | 1s error (%) | 87Sr/86Sr | 1s error (%) | ρ | Age Error | Ratio RSE | Ablation Length (sec) | Status | Note |
| 1 | 50 | K-feldspar | 17.0 | 0.70% | 1.06 | 0.49% | 0.54 | 1.25% | 1.41% | 65 | ||
| 2 | 50 | K-feldspar | 17.9 | 1.68% | 1.06 | 0.72% | 0.75 | 1.40% | 2.02% | 65 | ||
| 3 | 50 | K-feldspar | 20.4 | 1.63% | 1.12 | 0.79% | 0.51 | 1.87% | 2.42% | 25 | ||
| 4 | 50 | K-feldspar | 15.6 | 1.31% | 1.03 | 0.53% | 0.69 | 1.22% | 1.62% | 65 | ||
| 5 | 50 | K-feldspar | 16.8 | 0.94% | 1.05 | 0.51% | 0.45 | 1.33% | 1.46% | 50 | ||
| 6 | 50 | K-feldspar | 13.8 | 0.73% | 1.00 | 0.72% | 0.67 | 2.03% | 2.07% | 35 | ||
| 7 | 50 | K-feldspar | 17.3 | 0.86% | 1.07 | 0.63% | 0.64 | 1.43% | 1.91% | 40 | ||
| 8 | 50 | K-feldspar | 15.0 | 0.64% | 1.02 | 0.67% | 0.52 | 1.91% | 1.96% | 30 | ||
| 9 | 50 | K-feldspar | 13.0 | 0.91% | 0.981 | 0.53% | 0.31 | 1.88% | 1.57% | 40 | ||
| 10 | 50 | K-feldspar | 14.3 | 0.71% | 1.00 | 0.47% | 0.63 | 1.24% | 1.32% | 60 | ||
| 11 | 50 | K-feldspar | 11.7 | 0.77% | 0.962 | 0.54% | 0.57 | 1.74% | 1.58% | 40 | ||
| 12 | 50 | K-feldspar | 14.7 | 0.90% | 1.00 | 0.53% | 0.55 | 1.52% | 1.63% | 55 | ||
| 13 | 50 | K-feldspar | 15.8 | 1.52% | 1.03 | 0.64% | 0.67 | 1.49% | 1.86% | 65 | ||
| 14 | 50 | K-feldspar | 32.9 | 2.43% | 1.29 | 1.06% | 0.79 | 1.50% | 3.13% | 30 | rejected | unstable ablation, age mix? |
| 15 | 50 | K-feldspar | 37.0 | 62.90% | n.d. | n.d. | 3.99 | 88.04% | 66.99% | 5 | rejected | short ablation |
| 16 | 50 | K-feldspar | 30.1 | 25.84% | 1.32 | 36.36% | −1.06 | 78.49% | 63.24% | 5 | rejected | short ablation |
| 17 | 50 | Albite | 3.01 | 0.87% | 0.771 | 0.39% | 0.27 | 12.49% | 1.11% | 30 | mineral sample from 185m | |
| 18 | 50 | Albite | 3.59 | 0.81% | 0.781 | 0.30% | 0.19 | 6.87% | 0.87% | 55 | mineral sample from 185m | |
| 19 | 50 | Albite | 4.19 | 0.93% | 0.783 | 0.39% | 0.25 | 8.07% | 1.13% | 40 | mineral sample from 185m | |
| 20 | 50 | Albite | 1.16 | 15.86% | 0.773 | 1.61% | −0.11 | 47.10% | 16.29% | 65 | rejected | high 87Rb/86Sr errors |
| 21 | 50 | Albite | 3.72 | 7.93% | 0.805 | 2.32% | 0.47 | 35.30% | 6.16% | 30 | rejected | high 87Rb/86Sr errors |
| 22 | 50 | Albite | 3.10 | 8.69% | 0.796 | 1.77% | 0.28 | 47.17% | 4.36% | 30 | rejected | high 87Rb/86Sr errors |
| Sample: 185m | ||||||||||||
| Spot No. | Spot Size (μm) | Mineral | 87Rb/86Sr | 1s Error (%) | 87Sr/86Sr | 1s Error (%) | ρ | Age Error | Ratio RSE | Ablation Length (sec) | Status | Note |
| 23 | 50 | K-feldspar | 241 | 1.24% | 1.93 | 1.21% | 0.87 | 1.01% | 3.62% | 65 | ||
| 24 | 50 | K-feldspar | 263 | 1.92% | 2.06 | 1.69% | 0.86 | 1.36% | 5.33% | 50 | ||
| 25 | 50 | K-feldspar | 158 | 1.43% | 1.57 | 0.99% | 0.82 | 1.03% | 2.90% | 60 | ||
| 26 | 50 | K-feldspar | 203 | 2.22% | 1.73 | 1.74% | 0.93 | 1.35% | 4.94% | 60 | ||
| 27 | 50 | K-feldspar | 212 | 1.51% | 1.83 | 1.34% | 0.87 | 1.19% | 3.93% | 60 | ||
| 28 | 50 | K-feldspar | 222 | 1.41% | 1.84 | 1.08% | 0.81 | 1.06% | 3.15% | 65 | ||
| 29 | 50 | K-feldspar | 169 | 1.34% | 1.61 | 1.20% | 0.85 | 1.24% | 3.42% | 60 | ||
| 30 | 50 | K-feldspar | 166 | 1.37% | 1.57 | 1.19% | 0.80 | 1.37% | 3.60% | 60 | ||
| 31 | 50 | K-feldspar | 169 | 1.52% | 1.63 | 1.59% | 0.75 | 1.85% | 4.31% | 60 | ||
| 32 | 50 | K-feldspar | 546 | 2.60% | 3.17 | 2.46% | 0.95 | 1.07% | 7.34% | 60 | rejected | unstable ablation, high errors |
| 33 | 50 | K-feldspar | 182 | 1.78% | 1.66 | 1.36% | 0.90 | 1.15% | 4.11% | 60 | ||
| 34 | 50 | Calcite | - | - | 0.752 | 0.68% | 0.17 | - | - | 55 | ||
| 35 | 50 | Calcite | - | - | 0.745 | 1.26% | −0.39 | - | - | 15 | ||
| 36 | 50 | Calcite | - | - | 0.759 | 2.24% | −0.11 | - | - | 25 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillberg, M.; Ivarsson, M.; Drake, H.; Whitehouse, M.J.; Kooijman, E.; Schmitt, M. Re-Evaluating the Age of Deep Biosphere Fossils in the Lockne Impact Structure. Geosciences 2019, 9, 202. https://doi.org/10.3390/geosciences9050202
Tillberg M, Ivarsson M, Drake H, Whitehouse MJ, Kooijman E, Schmitt M. Re-Evaluating the Age of Deep Biosphere Fossils in the Lockne Impact Structure. Geosciences. 2019; 9(5):202. https://doi.org/10.3390/geosciences9050202
Chicago/Turabian StyleTillberg, Mikael, Magnus Ivarsson, Henrik Drake, Martin J. Whitehouse, Ellen Kooijman, and Melanie Schmitt. 2019. "Re-Evaluating the Age of Deep Biosphere Fossils in the Lockne Impact Structure" Geosciences 9, no. 5: 202. https://doi.org/10.3390/geosciences9050202
APA StyleTillberg, M., Ivarsson, M., Drake, H., Whitehouse, M. J., Kooijman, E., & Schmitt, M. (2019). Re-Evaluating the Age of Deep Biosphere Fossils in the Lockne Impact Structure. Geosciences, 9(5), 202. https://doi.org/10.3390/geosciences9050202