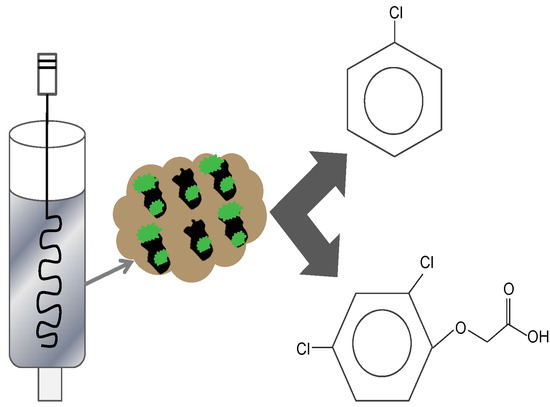
Fast Aqueous Biodegradation of Highly-Volatile Organic Compounds in a Novel Anaerobic Reaction Setup
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Adsorption Experiments
2.3. Experimental Set-up for Anaerobic Biodegradation
2.4. Analytical Methods
3. Results and Discussion
3.1. Adsorption Isotherms
3.2. Biodegradation of Chlorinated Compounds
3.3. Modelling CB and 2,4-D Degradation in USPBRs
3.3.1. Reaction Rate
3.3.2. Kinetic Models
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2014, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.; Mestre, A.S.; Andrade, M.; Ania, C.O. Ibuprofen in the aquatic environment: Occurrence, ecotoxicity and water remediation technologies. In Ibuprofen: Clinical Pharmacology, Medical Uses and Adverse Effects; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 1–84. ISBN 9781626186590. [Google Scholar]
- USEPA List of Priority Pollutants. Available online: http://water.epa.gov/drink/contaminants/index.cfm#List (accessed on 25 October 2018).
- Council, E. Directive 2008/105/EC of the European Parliament and of the Council of 16 december 2008 on environmental quality standards in the field of water policy. Off. J. Eur. Union L 2008, 348, 84–97. [Google Scholar]
- De Amarante, O.P.; Brito, N.M.; Dos Santos, T.C.R.; Nunes, G.S.; Ribeiro, M.L. Determination of 2,4-dichlorophenoxyacetic acid and its major transformation product in soil samples by liquid chromatographic analysis. Talanta 2003, 60, 115–121. [Google Scholar] [CrossRef]
- USEPA 2,4-Dichlorophenoxyacetic Acid (2,4-D) Chemical Summary. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P100BNRV.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery (accessed on 25 October 2018).
- USEPA 2,4-D R.E.D Facts. Available online: http://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000D2LZ.txt (accessed on 25 October 2018).
- Badellino, C.; Rodrigues, C.A.; Bertazzoli, R. Oxidation of herbicides by in situ synthesized hydrogen peroxide and fenton’s reagent in an electrochemical flow reactor: Study of the degradation of 2,4-dichlorophenoxyacetic acid. J. Appl. Electrochem. 2007, 37, 451–459. [Google Scholar] [CrossRef]
- Fontmorin, J.-M.; Huguet, S.; Fourcade, F.; Geneste, F.; Floner, D.; Amrane, A. Electrochemical oxidation of 2,4-dichlorophenoxyacetic acid: Analysis of by-products and improvement of the biodegradability. Chem. Eng. J. 2012, 195–196, 208–217. [Google Scholar] [CrossRef]
- Hoover, D.G.; Borgonovi, G.E.; Jones, S.H.; Alexander, M. Anomalies in mineralization of low concentrations of organic compounds in lake water and sewage. Appl. Environ. Microbiol. 1986, 51, 226–232. [Google Scholar] [PubMed]
- Brillas, E. Mineralization of 2,4-D by advanced electrochemical oxidation processes. Water Res. 2000, 34, 2253–2262. [Google Scholar] [CrossRef]
- Malcolm, H.M.; Howe, P.D.; Dobson, S. Chlorobenzenes Other Than Hexachlorobenzene: Environmental Aspects; World Health Organization: Geneva, Switzerland, 2004; ISBN 924153060X. [Google Scholar]
- Oonnittan, A.; Shrestha, R.A.; Sillanpää, M. Removal of hexachlorobenzene from soil by electrokinetically enhanced chemical oxidation. J. Hazard. Mater. 2009, 162, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Braeckevelt, M.; Reiche, N.; Trapp, S.; Wiessner, A.; Paschke, H.; Kuschk, P.; Kaestner, M. Chlorobenzene removal efficiencies and removal processes in a pilot-scale constructed wetland treating contaminated groundwater. Ecol. Eng. 2011, 37, 903–913. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R. Microbial degradation of chlorinated benzenes. Biodegradation 2007, 19, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, J.S.; Chen, Z.Y.; Wu, M.H.; Horii, Y.; Ohura, T.; Kannan, K. Chlorinated polycyclic aromatic hydrocarbons in urban surface dust and soil of Shanghai, China. Adv. Mater. Res. 2012, 610–613, 2989–2994. [Google Scholar] [CrossRef]
- Chary, N.S.; Herrera, S.; Gómez, M.J.; Fernández-Alba, A.R. Parts per trillion level determination of endocrine-disrupting chlorinated compounds in river water and wastewater effluent by stir-bar-sorptive extraction followed by gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2012, 404, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; McGrath, S.; Jones, K. The chlorobenzene content of archived sewage sludges. Sci. Total Environ. 1992, 121, 159–175. [Google Scholar] [CrossRef]
- USEPA OPPT Chemical Fact Sheets. Chlorobenzene. Available online: http://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1000ID3.txt (accessed on 25 October 2018).
- Burns, M.; Sublette, K.L.; Sobieraj, J.; Ogles, D.; Koenigsberg, S. Concurrent and complete anaerobic reduction and microaerophilic degradation of mono-, di-, and trichlorobenzenes. Remediat. J. 2013, 23, 37–53. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mostafapour, F.K.; Faridi, H.; Farzadkia, M.; Sohrabi, A. Removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous environments using single-walled carbon nanotubes. Heal. Scope 2013, 2, 39–46. [Google Scholar] [CrossRef]
- Farré, M.; Ferrer, I.; Ginebreda, A.; Figueras, M.; Olivella, L.; Tirapu, L.; Vilanova, M.; Barceló, D. Determination of drugs in surface water and wastewater samples by liquid chromatography–mass spectrometry: Methods and preliminary results including toxicity studies with Vibrio fischeri. J. Chromatogr. A 2001, 938, 187–197. [Google Scholar] [CrossRef]
- García-Martínez, Y.; Bengoa, C.; Stüber, F.; Fortuny, A.; Font, J.; Fabregat, A. Biodegradation of acid orange 7 in an anaerobic–aerobic sequential treatment system. Chem. Eng. Process. Process Intensif. 2015, 94, 99–104. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Rocher, V.; Gilbert-Pawlik, S.; Geara-Matta, D.; Moilleron, R.; Chebbo, G. Biofiltration vs. conventional activated sludge plants: What about priority and emerging pollutants removal? Environ. Sci. Pollut. Res. Int. 2014, 21, 5379–5390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, S.P.; Dantas, R.F.; Esplugas Vidal, S.; Sans Mazón, C.; Dezotti, M. Influence of EfOM on the oxidation of micropollutants by ozone and UV/H2O2 in secondary effluents. J. Environ. Sci. Eng. 2011, 5, 789–798. [Google Scholar]
- Girardi, C.; Nowak, K.M.; Carranza-Diaz, O.; Lewkow, B.; Miltner, A.; Gehre, M.; Schäffer, A.; Kästner, M. Microbial degradation of the pharmaceutical ibuprofen and the herbicide 2,4-D in water and soil-use and limits of data obtained from aqueous systems for predicting their fate in soil. Sci. Total Environ. 2013, 444, 32–42. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Müller, J.; Petrovic, M.; Barceló, D.; Knepper, T.P. Biodegradation studies of selected priority acidic pesticides and diclofenac in different bioreactors. Environ. Pollut. 2006, 144, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Devine, C.E.; Nelson, J.; Sherwood Lollar, B.; Zinder, S.; Edwards, E.A. Anaerobic conversion of chlorobenzene and benzene to CH4 and CO2 in bioaugmented microcosms. Environ. Sci. Technol. 2013, 47, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Samaras, V.G.; Stasinakis, A.S.; Thomaidis, N.S.; Mamais, D.; Lekkas, T.D. Fate of selected emerging micropollutants during mesophilic, thermophilic and temperature co-phased anaerobic digestion of sewage sludge. Bioresour. Technol. 2014, 162, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Savant, D.V.; Abdul-Rahman, R.; Ranade, D.R. Anaerobic degradation of adsorbable organic halides (AOX) from pulp and paper industry wastewater. Bioresour. Technol. 2006, 97, 1092–10104. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Geetanjali Basak, L.V.; Salam, J.A.; Evy Alice Abigail, M. Application of biofilms on remediation of pollutants—An overview. J. Microbiol. Biotechnol. Res. 2012, 2, 783–790. [Google Scholar]
- Andersson, S.; Kuttuva Rajarao, G.; Land, C.J.; Dalhammar, G. Biofilm formation and interactions of bacterial strains found in wastewater treatment systems. FEMS Microbiol. Lett. 2008, 283, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingender, J.; Flemming, H.-C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Paul, D.; Jain, R.K. Biofilms: Implications in bioremediation. Trends Microbiol. 2006, 14, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Aroua, M.; Daud, W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Herzberg, M.; Dosoretz, C.G.; Kuhn, J.; Klein, S.; Green, M. Visualization of active biomass distribution in a BGAC fluidized bed reactor using GFP tagged Pseudomonas putida F1. Water Res. 2006, 40, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Mezohegyi, G.; Kolodkin, A.; Castro, U.I.; Bengoa, C.; Stuber, F.; Font, J.; Fabregat, A.; Fortuny, A. Effective anaerobic decolorization of azo dye Acid Orange 7 in continuous upflow packed-bed reactor using biological activated carbon system. Ind. Eng. Chem. Res. 2007, 46, 6788–6792. [Google Scholar] [CrossRef]
- Athalathil, S.; Stüber, F.; Bengoa, C.; Font, J.; Fortuny, A.; Fabregat, A. Characterization and performance of carbonaceous materials obtained from exhausted sludges for the anaerobic biodecolorization of the azo dye Acid Orange II. J. Hazard. Mater. 2014, 267, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mezohegyi, G.; Bengoa, C.; Stuber, F.; Font, J.; Fabregat, A.; Fortuny, A. Novel bioreactor design for decolourisation of azo dye effluents. Chem. Eng. J. 2008, 143, 293–298. [Google Scholar] [CrossRef]
- Grueiro Noche, G.; Fernández Laespada, M.E.; Pérez Pavón, J.L.; Moreno Cordero, B.; Muniategui Lorenzo, S. Determination of chlorobenzenes in water samples based on fully automated microextraction by packed sorbent coupled with programmed temperature vaporization-gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 6739–6748. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jury, W.A.; Wagenet, R.J.; Flury, M. Dependence of pesticide degradation on sorption: Nonequilibrium model and application to soil reactors. J. Contam. Hydrol. 2000, 43, 45–62. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon N. Y. 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lai, C.-W.; Su, T.-Y. Adsorption of bisphenol-A from aqueous solution onto minerals and carbon adsorbents. J. Hazard. Mater. 2006, 134, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Mansouri, H.; Carmona, R.J.; Gomis-Berenguer, A.; Souissi-Najar, S.; Ouederni, A.; Ania, C.O. Competitive adsorption of ibuprofen and amoxicillin mixtures from aqueous solution on activated carbons. J. Colloid Interface Sci. 2015, 449, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Garg, V.; Sapag, K.; Lago, R.M. Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon N. Y. 2002, 40, 2177–2183. [Google Scholar] [CrossRef]
- Salman, J.M.; Al-Saad, K.A. Adsorption of 2,4-dichlorophenoxyacetic acid onto date seeds activated carbon: Equilibrium, kinetic and thermodynamic studies. Int. J. Chem. Sci. 2012, 10, 677–690. [Google Scholar]
- Elefsiniotis, P.; Chin, H.; Singhal, N. Biodegradation of 2,4-dicholophenoxyacetic acid using an acidogenic anaerobic sequencing batch reactor. J. Environ. Eng. Sci. 2005, 4, 57–63. [Google Scholar] [CrossRef]
- Mangat, S.S.; Elefsiniotis, P. Biodegradation of the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) in sequencing batch reactors. Water Res. 1999, 33, 861–867. [Google Scholar] [CrossRef]
- Scholz, M.; Martin, R.J. Ecological equilibrium on biological activated carbon. Water Res. 1997, 31, 2959–2968. [Google Scholar] [CrossRef]
- Emanuelsson, E.A.C.; Baptista, I.I.R.; Mantalaris, A.; Livingston, A.G. Strain stability in biological systems treating recalcitrant organic compounds. Biotechnol. Bioeng. 2005, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Jechorek, M.; Wendlandt, K.-D.; Beck, M. Cometabolic degradation of chlorinated aromatic compounds. J. Biotechnol. 2003, 102, 93–98. [Google Scholar] [CrossRef]
- Wang, J.; Mei, Y.; Liu, C.; Chen, J. Chlorobenzene degradation by electro-heterogeneous catalysis in aqueous solution: Intermediates and reaction mechanism. J. Environ. Sci. 2008, 20, 1306–1311. [Google Scholar] [CrossRef]
- Moreira, I.S.; Amorim, C.L.; Carvalho, M.F.; Castro, P.M.L. Co-metabolic degradation of chlorobenzene by the fluorobenzene degrading wild strain Labrys portucalensis. Int. Biodeterior. Biodegrad. 2012, 72, 76–81. [Google Scholar] [CrossRef]
- González, A.J.; Gallego, A.; Gemini, V.L.; Papalia, M.; Radice, M.; Gutkind, G.; Planes, E.; Korol, S.E. Degradation and detoxification of the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) by an indigenous Delftia sp. strain in batch and continuous systems. Int. Biodeterior. Biodegrad. 2012, 66, 8–13. [Google Scholar] [CrossRef]
- Quan, X.; Tang, H.; Ma, J. Effects of gene augmentation on the removal of 2,4-dichlorophenoxyacetic acid in a biofilm reactor under different scales and substrate conditions. J. Hazard. Mater. 2011, 185, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Vroumsia, T.; Steiman, R.; Seiglemurandi, F.; Benoitguyod, J. Fungal bioconversion of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4-dichlorophenol (2,4-DCP). Chemosphere 2005, 60, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Elefsiniotis, P.; Wareham, D.G. Biodegradation of industrial-strength 2,4-dichlorophenoxyacetic acid wastewaters in the presence of glucose in aerobic and anaerobic sequencing batch reactors. Environ. Technol. 2013, 34, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Quintelas, C.; Silva, B.; Figueiredo, H.; Tavares, T. Removal of organic compounds by a biofilm supported on GAC: Modelling of batch and column data. Biodegradation 2010, 21, 379–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-Garcı́a, M.; Moreno-Castilla, C. Bioadsorption of Pb(II), Cd(II), and Cr(VI) on activated carbon from aqueous solutions. Carbon N. Y. 2003, 41, 323–330. [Google Scholar] [CrossRef]
- Van der Zee, F.P.; Villaverde, S. Combined anaerobic-aerobic treatment of azo dyes-A short review of bioreactor studies. Water Res. 2005, 39, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, F.P.; Bisschops, I.A.E.; Lettinga, G.; Field, J.A. Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes. Environ. Sci. Technol. 2003, 37, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.K.; Sundaramurthy, J.; Balomajumder, C. Kinetics of the removal of mono-chlorobenzene vapour from waste gases using a trickle bed air biofilter. J. Hazard. Mater. 2006, 137, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Chen, J.; Xu, B.; Chu, G.; Chen, J. Performance and microbial analysis of two different inocula for the removal of chlorobenzene in biotrickling filters. Chem. Eng. J. 2016, 284, 174–181. [Google Scholar] [CrossRef]
- Wang, S.-J.; Loh, K.-C. Modeling the role of metabolic intermediates in kinetics of phenol biodegradation. Enzyme Microb. Technol. 1999, 25, 177–184. [Google Scholar] [CrossRef]







| Compound | k1 (mmol gcat−1 min−1) | k2 (mmol L−1) | σ a |
|---|---|---|---|
| CB | 1.28 | 0.32 | 0.003 |
| 2,4-D | 0.098 | 0.00014 | 0.05 |
| k1 (mmol gcat−1 min−1) | k2 (mmol L−1) | ki (mmol L−1) | σ |
|---|---|---|---|
| 0.55 | 0.19 | 0.071 | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martínez, Y.; Chirinos, J.; Bengoa, C.; Stüber, F.; Font, J.; Fortuny, A.; Fabregat, A. Fast Aqueous Biodegradation of Highly-Volatile Organic Compounds in a Novel Anaerobic Reaction Setup. Environments 2018, 5, 115. https://doi.org/10.3390/environments5110115
García-Martínez Y, Chirinos J, Bengoa C, Stüber F, Font J, Fortuny A, Fabregat A. Fast Aqueous Biodegradation of Highly-Volatile Organic Compounds in a Novel Anaerobic Reaction Setup. Environments. 2018; 5(11):115. https://doi.org/10.3390/environments5110115
Chicago/Turabian StyleGarcía-Martínez, Yonhara, Judith Chirinos, Christophe Bengoa, Frank Stüber, Josep Font, Agustí Fortuny, and Azael Fabregat. 2018. "Fast Aqueous Biodegradation of Highly-Volatile Organic Compounds in a Novel Anaerobic Reaction Setup" Environments 5, no. 11: 115. https://doi.org/10.3390/environments5110115
APA StyleGarcía-Martínez, Y., Chirinos, J., Bengoa, C., Stüber, F., Font, J., Fortuny, A., & Fabregat, A. (2018). Fast Aqueous Biodegradation of Highly-Volatile Organic Compounds in a Novel Anaerobic Reaction Setup. Environments, 5(11), 115. https://doi.org/10.3390/environments5110115