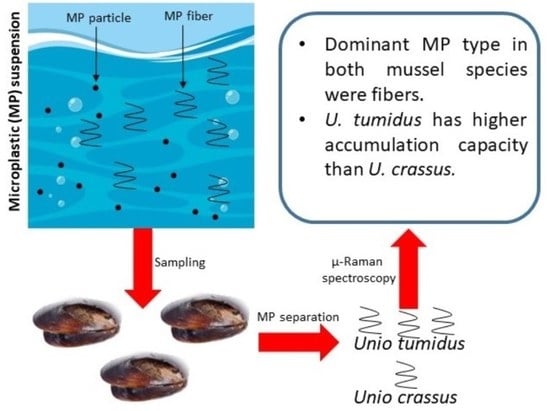
Comparison of Freshwater Mussels Unio tumidus and Unio crassus as Biomonitors of Microplastic Contamination of Tisza River (Hungary)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals and Reagents
2.3. Sample Preaparation
2.4. Analysis of Residues via Optical Microscopy and Raman Spectrometry
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wardlaw, C.; Prosser, R.S. Investigation of Microplastics in Freshwater Mussels (Lasmigona costata) From the Grand River Watershed in Ontario, Canada. Water Air Soil Pollut. 2020, 231, 405. [Google Scholar] [CrossRef]
- Kinjo, A.; Mizukawa, K.; Takada, H.; Inoue, K. Size-dependent elimination of ingested microplastics in the Mediterranean mussel Mytilus galloprovincialis. Mar. Pollut. Bull. 2019, 149, 110512. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Ma, L.S.; Lin, L.; Ni, Z.X.; Xu, X.R.; Shi, H.H.; Yan, Y.; Zheng, M.G.; Rittschof, D. Microplastics in oysters Saccostrea cucullata along the Pearl River Estuary, China. Environ. Pollut. 2018, 236, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Øysæd, B.K.; Fabi, G. First occurrence and composition assessment of microplastics in native mussels collected from coastal and offshore areas of the northern and central Adriatic Sea. Environ. Sci. Pollut. Res. 2019, 26, 24407–24416. [Google Scholar] [CrossRef]
- Alfaro-Núñez, A.; Astorga, D.; Cáceres-Farías, L.; Bastidas, L.; Villegas, S.C.; Macay, C.K.; Christensen, H.J. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Sci. Rep. 2021, 11, 6424. [Google Scholar] [CrossRef]
- Ward, E.J.; Zhao, S.; Holohan, B.A.; Mladinich, K.M.; Griffin, T.W.; Wozniak, J.; Shumway, S.E. Selective Ingestion and Egestion of Plastic Particles by the Blue Mussel (Mytilus edulis) and Eastern Oyster (Crassostrea virginica): Implications for Using Bivalves as Bioindicators of Microplastic Pollution. Environ. Sci. Technol. 2019, 53, 8776–8784. [Google Scholar] [CrossRef]
- Zantis, J.L.; Carroll, L.E.; Nelms, E.S.; Bosker, T. Marine mammals and microplastics: A systematic review and call for standardisation. Envrion. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef]
- Meaza, I.; Toyoda, H.J.; Wise Sr, P.J. Microplastics in Sea Turtles, Marine Mammals and Humans: A One Environmental Health Perspective. Front. Environ. Sci. 2021, 8, 575614. [Google Scholar] [CrossRef]
- Costa, E.; Piazza, V.; Lavorano, S.; Faimali, M.; Garaventa, F.; Gambardella, C. Trophic Transfer of Microplastics From Copepods to Jellyfish in the Marine Environment. Front. Environ. Sci. 2020, 8, 571732. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Vandermeersch, G.; Van Cauwenberghe, L.; Janssen, C.R.; Marques, A.; Granby, K.; Fait, G.; Kotterman, M.J.J.; Diogène, J.; Bekaert, K.; Robbens, J.; et al. A critical view on microplastic quantification in aquatic organisms. Environ. Res. 2015, 143, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; van Werven, B.; van Oyen, A.; Meijboom, A.; Bravo Rebolledo, E.L.; van Franeker, J.A. The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar. Pollut. Bull. 2017, 115, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bråte, I.L.N.; Hurley, R.; Iversen, K.; Beyer, J.; Thomas, K.V.; Steindal, C.C.; Green, W.N.; Olsen, M.; Lusher, A. Mytilus spp. as sentinels for monitoring microplastic pollution in Norwegian coastal waters: A qualitative and quantitative study. Environ. Pollut. 2018, 243, 383–393. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-Bonaca, M. Perspectives on using marine species as bioindicators of plastic pollution. Mar. Pollut. Bull. 2018, 137, 209–221. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Rotchell, J.M.; Shen, X.; Li, Q.; Zhu, J. Where are we? Towards an understanding of the selective accumulation of microplastics in mussels. Environ. Pollut. 2021, 286, 117543. [Google Scholar] [CrossRef]
- Cera, A.; Scalici, M. Freshwater wild biota exposure to microplastics: A global perspective. Ecol. Evol. 2021, 11, 9904–9916. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Staichak, G.; Ferreira-Jr, A.L.; Moreschi Silva, A.C.; Girard, P.; Callil, C.T.; Christo, S.W. Bivalves with potential for monitoring microplastics in South America. Case Stud. Chem. Environ. Eng. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Egede, P.P.; Barreiro Saavedra, I. Feeding Behaviour of the Mussel, Mytilus edulis: New Observations, with a Minireview of Current Knowledge. J. Mar. Biol. 2011, 2011, 312459. [Google Scholar] [CrossRef]
- Naidu, S.A. Preliminary study and first evidence of presence of microplastics and colorants in green mussel, Perna viridis (Linnaeus, 1758), from southeast coast of India. Mar. Pollut. Bull. 2019, 140, 416–422. [Google Scholar] [CrossRef]
- Tankersley, R.A.; Dimock Jnr, R.V. The effect of larval brooding on the filtration rate and particle-retention efficiency of Pyganodon cataracta (Bivalvia: Unionidae). Can. J. Zool. 1993, 71, 1934–1944. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, G.; Wang, W.; Wang, J. Microplastic pollution research methodologies, abundance, characteristics and risk assessments for aquatic biota in China. Environ. Pollut. 2020, 266, 115098. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Hossain, S.M.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Ward, E.J.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthrop. Coast. 2019, 2, 39–49. [Google Scholar] [CrossRef]
- Jørgensen, C.; Kørboe, T.; Møhlenberg, F.; Riisgård, H. Ciliary and mucus-net filter feeding, with special reference to fluid mechanical Characteristics. Mar. Ecol. Prog. Ser. 1984, 15, 283–292. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Shumway, S.E.; Wikfors, G.H.; Pales-Espinosa, E.; Allam, B. Effects of particle surface properties on feeding selectivity in the eastern oyster Crassostrea virginica and the blue mussel Mytilus edulis. J. Exp. Mar. Biol. Ecol. 2013, 446, 320–327. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Frink, A.; Shumway, S.E. Effects of Surface Properties on Particle Capture by Two Species of Suspension-Feeding Bivalve Molluscs. Am. Malacol. Bull. 2017, 35, 181–188. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Shumway, S.E. Selective Capture and Ingestion of Particles by Suspension-Feeding Bivalve Molluscs: A Review. J. Shellfish Res. 2018, 37, 727–746. [Google Scholar] [CrossRef]
- Ward, E.J. Biodynamics of Suspension-Feeding in Adult Bivalve Molluscs: Particle Capture, Processing, and Fate. Invertebr. Biol. 1996, 115, 218–231. [Google Scholar] [CrossRef]
- Ward, E.J.; Shumway, S.E. Separating the grain from the chaff: Particle selection in suspension- and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300, 83–130. [Google Scholar] [CrossRef]
- Berglund, E.; Fogelberg, V.; Nilsson, P.A.; Hollander, J. Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Sci. Total Environ. 2019, 697, 134192. [Google Scholar] [CrossRef] [PubMed]
- Schessl, M.; Johns, C.; Ashpole, S.L. Microbeads in sediment, dreissenid mussels, and anurans in the littoral zone of the upper St. Lawrence River, New York. Pollution 2019, 5, 41–52. [Google Scholar] [CrossRef]
- McCoy, K.A.; Hodgson, D.J.; Clark, P.F.; Morritt, D. The effects of wet wipe pollution on the Asian clam, Corbicula fluminea (Mollusca: Bivalvia) in the River Thames, London. Environ. Pollut. 2020, 264, 114577. [Google Scholar] [CrossRef]
- Doucet, C.V.; Labaj, A.L.; Kurek, J. Microfiber Content in Freshwater Mussels from Rural Tributaries of the Saint John River, Canada. Water Air Soil Pollut. 2021, 232, 32. [Google Scholar] [CrossRef]
- Hoellein, T.; Rovegno, C.; Uhrin, A.V.; Johnson, E.; Herring, C. Microplastics in Invasive Freshwater Mussels (Dreissena sp.): Spatiotemporal Variation and Occurrence With Chemical Contaminants. Front. Mar. Sci. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Kiss, T.; Fórián, S.; Szatmári, G.; Sipos, G. Spatial distribution of microplastics in the fluvial sediments of a transboundary river—A case study of the Tisza River in Central Europe. Sci. Total Environ. 2021, 785, 147306. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef]
- Zając, K.; Florek, J.; Zając, T.; Adamski, P.; Bielański, W.; Ćmiel, A.M.; Klich, M.; Lipińska, M.A. On the reintroduction of the endangered thick-shelled river mussel Unio crassus: The importance of the river’s longitudinal profile. Sci. Total Environ. 2018, 624, 273–282. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 10 June 2022).
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- McMahon, F.B.; Bogan, E.A. Ecology and Classification of North American Freshwater Invertebrates, 1st ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 331–429. [Google Scholar] [CrossRef]
- Kryger, J.; Riisgård, H.U. Filtration rate capacities in 6 species of European freshwater bivalves. Oecologia 1988, 77, 34–38. [Google Scholar] [CrossRef]
- Weber, A.; Jeckel, N.; Weil, C.; Umbach, S.; Brennholt, N.; Reifferscheid, G.; Wagner, M. Ingestion and Toxicity of Polystyrene Microplastics in Freshwater Bivalves. Environ. Toxicol. Chem. 2021, 40, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.C.; Sembiring, E.; Muntalif, B.S.; Suendo, V. Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia). Chemosphere 2019, 224, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef]
- De Falco, F.; Pace, D.E.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Carr, S.A. Sources and dispersive modes of micro-fibers in the environment. Integr. Environ. Asses. 2017, 13, 466–469. [Google Scholar] [CrossRef]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic fibers—Underestimated threat to aquatic organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef] [PubMed]



| Study Area | Bivalve Type | Abundance | Dominant Shape | Size | Dominant Color | Chemical Composition | Ref |
|---|---|---|---|---|---|---|---|
| Grand River, Canada | Mussel Lasmigona costata | 0–0.16 items/g 0–7 items/ind. | Fragments | 21–298 μm | White | PP-co-EP PP | [1] |
| Höje River, Sweden | Mussel Anodonta anatina | 4–142 plastic fibers/ind. | Fibers | N/A | Black | N/A | [33] |
| Saint John River, Canada | Mussel M. margaritifera L | 0–0.6 microfibers/g 0–10.9 microfibers/g | Microfibers | >100 μm | Blue | N/A | [31] |
| St. Lawrence River, USA | Mussel Dreissena polymorpha D. bugensis | N/A | Not found | N/A | N/A | N/A | [36] |
| Milwaukee River, USA | Mussel Dreissena sp. | 8.4 items/g 0.6 items/ind. | Fibers | N/A | Clear | Cotton natural Cellulose-based natural PET | [37] |
| Yangtze River, China | Asian clam Corbicula fluminea | 0.3–4.9 items/g 0.4–5.0 items/ind. | Fibers | 250–1000 μm | Blue | PET 33% PP 19% PE 9% | [4] |
| Thames River, UK | Asian clam Corbicula fluminea | 0–24 items/ind. | Fibers | N/A | N/A | PP 57% PE 9% Nylon 8% Polyallomer 8% PVP 6% Others 12% | [35] |
| Pearl River, China | Oyster Saccostrea cucullata | 1.5–7.2 items/g 1.4–7 items/ nd. | Fibers | <100 μm | Light color | PET 34% PP 19% Pe 14% PS 8% Cellophane 8% PVC 6% Polyamide 4% Expanded polystyrene 3% | [3] |
| Mussel | Site | Shell Length (mm) | Soft Tissue Wet Weight (g) | Number of Fibers/ind | Number of Fibers/g |
| Unio crassus | Tímár | 62 ± 5 | 10.94 ± 1.75 | 2.8 ± 0.5 a | 0.25 |
| Tokaj | 68 ± 3 | 9.85 ± 2.34 | 2.7 ± 0.5 a | 0.27 | |
| Csongrád | 64 ± 7 | 9.53 ± 2.72 | 4.9 ± 1.2 bc | 0.51 | |
| Szeged | 71 ± 4 | 12.82 ± 2.12 | 3.8 ± 0.8 ab | 0.29 | |
| Unio tumidus | Tímár | 67 ± 5 | 7.15 ± 1.74 | 5.2 ± 1.4 bcd | 0.72 |
| Tokaj | 63 ± 6 | 6.84 ± 1.33 | 6.0 ± 1.3 cd | 0.87 | |
| Csongrád | 70 ± 8 | 6.94 ± 2.18 | 7.2 ± 1.9 d | 1.03 | |
| Szeged | 77 ± 8 | 7.95 ± 2.33 | 7.1 ± 2.4 cd | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeshal, W.; Takács, A.; Aradi, L.; Sandil, S.; Dobosy, P.; Záray, G. Comparison of Freshwater Mussels Unio tumidus and Unio crassus as Biomonitors of Microplastic Contamination of Tisza River (Hungary). Environments 2022, 9, 122. https://doi.org/10.3390/environments9100122
Almeshal W, Takács A, Aradi L, Sandil S, Dobosy P, Záray G. Comparison of Freshwater Mussels Unio tumidus and Unio crassus as Biomonitors of Microplastic Contamination of Tisza River (Hungary). Environments. 2022; 9(10):122. https://doi.org/10.3390/environments9100122
Chicago/Turabian StyleAlmeshal, Wael, Anita Takács, László Aradi, Sirat Sandil, Péter Dobosy, and Gyula Záray. 2022. "Comparison of Freshwater Mussels Unio tumidus and Unio crassus as Biomonitors of Microplastic Contamination of Tisza River (Hungary)" Environments 9, no. 10: 122. https://doi.org/10.3390/environments9100122
APA StyleAlmeshal, W., Takács, A., Aradi, L., Sandil, S., Dobosy, P., & Záray, G. (2022). Comparison of Freshwater Mussels Unio tumidus and Unio crassus as Biomonitors of Microplastic Contamination of Tisza River (Hungary). Environments, 9(10), 122. https://doi.org/10.3390/environments9100122