Role of Altitude in Formation of Diatom Diversity of High Mountain Protected Glacier Lakes in the Kaçkar Mountains National Park, Rize, Turkey
Abstract
:1. Introduction
2. Materials and Methods
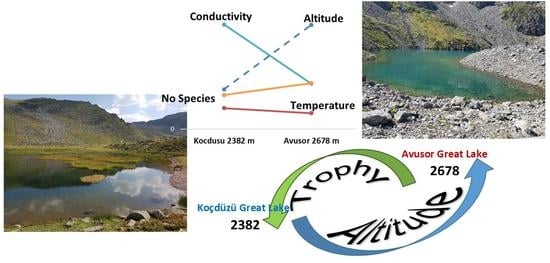
2.1. Study Area
2.2. Sampling and Laboratory Studies
3. Results
3.1. Physical and Chemical Analysis
3.2. Diatom Assemblages
3.3. Bioindicators Analysis
3.4. Comparative Floristic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bundi, U. Synthesis: Features of alpine waters and management concerns. In Alpine Waters, 1st ed.; Bundi, U., Ed.; Springer: Heidelberg, Germany, 2010; pp. 1–16. [Google Scholar]
- Winkler, D.E. Contemporary Human Impacts on Alpine Ecosystems: The Direct and Indirect Effects of Human-Induced Climate Change and Land Use, 1st ed.; Elsevier Publishing: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sommaruga, R. The role of solar UV radiation in the ecology of alpine lakes. J. Photochem. Photobiol. B Biol. 2001, 62, 35–42. [Google Scholar] [CrossRef]
- Tolotti, M. Phytoplanton and littoral epilithic diatoms in high mountain lakes of the Adamello-Brenta Regional Park (Trentino, Italy) and their relation to trophic status and acidification risk. J. Limnol. 2001, 60, 171–188. [Google Scholar] [CrossRef]
- Catalan, J.; Camerero, L.; Felip, M.; Pla, S.; Ventura, M. High mountain lakes: Extreme habitats and witnesses of environmental changes. Limnetica 2006, 25, 551–584. [Google Scholar] [CrossRef]
- Rautio, M.; Sorvari, S.; Korhola, A. Diatom and crustacean zooplankton communities, their seasonal variability and representation in the sediments of subarctic Lake Saanajärvi. J. Limnol. 2000, 59, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Medvedeva, L.A. Biodiversity of aquatic algal communities in the Sikhote-Alin biosphere reserve (Russia). Cryptogam. Algol. 2001, 22, 65–100. [Google Scholar] [CrossRef]
- Niyatbekov, T.; Barinova, S. Diatom species richness in algal flora of Pamir, Tajikistan. Eur. Sci. J. 2018, 14, 301–323. [Google Scholar] [CrossRef] [Green Version]
- Barinova, S.; Niyatbekov, T. Comparative analysis of diatom algae diversity in the Pamir Protected Lakes, Tajikistan. Int. J. Adv. Res. Bot. 2019, 5, 1–17. [Google Scholar] [CrossRef]
- Sigee, D.C. Freshwater Microbiology: Biodiversity and Dynamic Interactions of Microorganisms in the Aquatic Environment, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2005. [Google Scholar]
- Pouličková, A.; Hašler, P.; Lysákova, M.; Spears, B. The ecology of freshwater epipelic algae: An update. Phycologia 2008, 47, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Round, F.E. A Review and Methods for the Use of Epilithic Diatoms for Detecting and Monitoring Changes in River Water Quality, 1st ed.; HMSO: London, UK, 1993.
- Taş, B.; Yılmaz, Ö.; Kurt, I. Aşağı Melet Irmağı (Ordu), Türkiye)’nda su kalitesinin göstergesi olan diyatomeler. Türk Tarım-Gıda Bilim Teknol. Derg. 2015, 3, 610–616. (In Turkish) [Google Scholar] [CrossRef] [Green Version]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32000L0060 (accessed on 7 June 2022).
- Kazancı, N. Ephemeroptera Fauna (Insecta) of Gümüşhane, Erzurum, Erzincan, Artvin, Kars Provinces, 1st ed.; Inland Waters of Turkey Series V; Imaj Press: Ankara, Turkey, 2001. [Google Scholar]
- Kazancı, N. Records of Plecoptera (Insecta) species and affects of episodic acidification on physicochemical properties of their habitats in the Eastern Black Sea Region and Yeşilırmak River Basin. Rev. Hydrobiol. 2009, 2, 196–206. [Google Scholar]
- Kazancı, N. The records of Plecoptera (Insecta) species from Eastern part of Black Sea Region (Turkey). Rev. Hydrobiol. 2013, 6, 121–127. [Google Scholar]
- Ekingen, P.; Kazancı, N. Key to the family of Trichoptera (Insecta) larvae of Aksu Stream (Giresun, Turkey) in Eastern part of Black Sea Region. Rev. Hydrobiol. 2013, 6, 145–155. [Google Scholar]
- Türkmen, G.; Kazancı, N. Additional records of Ephemeroptera (Insecta) species from the Eastern part of Black Sea Region (Turkey). Rev. Hydrobiol. 2015, 8, 33–50. [Google Scholar]
- Polat, P.; Sunkar, M. The climatic characteristics of Rize and the trend analyses of long-term temperature and precipitation data around Rize. J. Int. Soc. Sci. 2017, 27, 1–23. [Google Scholar]
- Round, F.E. An investigation of two benthic algal communities in Malharm Tarn, Yorkshire. J. Ecol. 1953, 41, 174–197. [Google Scholar] [CrossRef]
- Sládečková, A. Limnological investigation methods for the periphyton (“Aufwuchs”) community. Bot. Rev. 1962, 28, 286–350. [Google Scholar] [CrossRef]
- Hustedt, F. Die Süswasser Flora Mitteleuropas Heft 10: Bacillariophyta (Diatomeae), 1st ed.; Gustav Fischer Verlag: Jena, Germany, 1930.
- Huber-Pestalozzi, G. Das Phytoplankton des Susswasers. Systematik und Biologie. In Die Binnengewasser, 16, 1st ed.; Thienemann, A., Ed.; E. Schweizerbart’sche Verlagsbuchhandlung (Nagele u. Obermiller): Stuttgart, Germany, 1942. [Google Scholar]
- Patrick, R.; Reimer, C.W. The Diatoms of the United States Vol 1. Fragilariaceae, Eunotiaceae, Achnanthaceae, Naviculacae, 1st ed.; Academy of Natural Science: Philadelphia, PA, USA, 1966. [Google Scholar]
- Patrick, R.; Reimer, C.W. The Diatoms of the United States Vol 2. Entomoneigaceae, Cymbellaceae, Gomphonemaceae, Epithemiaceae, 1st ed.; Academy of Natural Science: Philadelphia, PA, USA, 1975. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 1: Naviculaceae. In Süsswasserflora von Mitteleuropa 2/1, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1986. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 2: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa 2/2, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1988. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 3: Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa 2/3, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1991. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 4: Achnanthaceae. In Süsswasserflora von Mitteleuropa 2/4, 1st ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1991. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe, 1st ed.; Cantonati, M., Kelly, M.G., Lange-Bertalot, H., Eds.; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017. [Google Scholar]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: http://www.algaebase.org (accessed on 15 June 2022).
- Barinova, S. How to align and unify the cell counting of organisms for bioindication. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555–585. [Google Scholar] [CrossRef] [Green Version]
- Willis, J.C. Age and Area. A Study in Geographical Distribution and Origin of Species; Cambridge University Press: Cambridge, UK, 1922. [Google Scholar]
- Barinova, S. Systemic criteria for the analysis of alpha- and gamma-diversity of freshwater algae. Int. J. Environ. Sci. Nat. Resour. 2017, 4, 555–633. [Google Scholar] [CrossRef]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment, 1st ed.; Pilies Studio Publisher: Tel Aviv, Israel, 2006. (In Russian) [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Perspectives, 1st ed.; Publishing House of Haifa University: Haifa, Israel, 2019. (In Russian) [Google Scholar]
- Wessa, P. Pearson Correlation (v1.0.13) in Free Statistics Software (v1.2.1), Office for Research Development and Education. Available online: https://www.wessa.net/rwasp_correlation.wasp/ (accessed on 22 July 2022).
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Barinova, S. Algal Diversity Dynamics, Ecological Assessment, and Monitoring in the River Ecosystems of the Eastern Mediterranean, 1st ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Şahin, B.; Akar, B.; Barinova, S. Algal Flora and Ecology of the High Mountain Lakes in the Artabel Lakes Nature Park (Gümüşhane, Turkey), I–Bacillariophyta. Int. J. Adv. Res. Bot. 2019, 5, 25–37. [Google Scholar] [CrossRef]
- Şahin, B.; Akar, B.; Barınova, S. Cohabitant charophyte algal flora and its ecology in high-mountain lakes of the Artabel Lakes Nature Park (Gümüşhane, Turkey). Bot. Serbica 2020, 44, 11–25. [Google Scholar] [CrossRef]
- Barinova, S.; Kukhaleishvili, L.; Nevo, E.; Janelidze, Z. Diversity and ecology of algae in the Algeti National Park as a part of the Georgian system of protected areas. Turk. J. Bot. 2011, 35, 729–774. [Google Scholar] [CrossRef]
- Barinova, S.; Boboev, M.; Hisoriev, H. Freshwater algal diversity of the South-Tajik Depression in a high mountainous extreme environment. Turk. J. Bot. 2015, 39, 535–546. [Google Scholar] [CrossRef]
- Şahin, B.; Akar, B. Nine new records from high mountain lakes (Artabel Lakes Nature Park, Gümüşhane/Turkey) for the freshwater distom flora of Turkey. BioDiCon 2018, 11, 56–63. [Google Scholar]
- Sládeček, V. System of water quality from the biological point of view. Arch. Hydrobiol. 1973, 7, 1–218. [Google Scholar]
- Barinova, S. On the Classification of Water Quality from an Ecological Point of View. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Atıcı, T.; Tokatli, C. Algal Diversity and Water Quality Assessment with Cluster Analysis of Four Freshwater Lakes (Mogan, Abant, Karagöl and Poyrazlar) of Turkey. Wulfenia 2014, 21, 155–169. [Google Scholar]
- Barinova, S.; Gabyshev, V.; Boboev, M.; Kukhaleishvili, L.; Bilous, O. Algal Indication of Climatic Gradients. Am. J. Environ. Protection. Spec. Issue Appl. Ecol. Probl. Innov. 2015, 4, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Poikane, S.; Kelly, M.; Cantonati, M. Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total Environ. 2016, 568, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Dedić, A.; Gerhardt, A.; Kelly, M.G.; Stanić-Koštroman, S.; Šiljeg, M.; Kalamujić Stroil, B.; Kamberović, J.; Mateljak, Z.; Pešić, V.; Vučković, I.; et al. Innovative methods and approaches for WFD: Ideas to fill knowledge gaps in science and policy. Water Solut. 2020, 3, 30–42. [Google Scholar]
- Robinson, C.T.; Kawecka, B. Benthic diatoms of an alpine stream/lake network in Switzerland. Aquat. Sci. 2005, 67, 492–506. [Google Scholar] [CrossRef]
- Levkov, Z.; Krstic, S.; Nakov, T.; Melovski, L. Diatom assemblages on Shara and Nidze Mountains, Macedonia. Nova Hedwig. 2005, 81, 501–537. [Google Scholar] [CrossRef]
- Şahin, B. Epipelic and epilithic algae of Dağbaşı Lake (Rize-Turkey). Turk. J. Bot. 2001, 25, 187–194. [Google Scholar]
- Şahin, B.; Akar, B. Epipelic and epilithic algae of Küçükgöl Lake (Gümüşhane, Turkey). Turk. J. Biol. 2005, 29, 57–63. [Google Scholar]
- Şahin, B. Species composition and diversity of epipelic algae in Limni Lake (Gümüşhane, Turkey). Acta Bot. Hung. 2008, 50, 397–405. [Google Scholar] [CrossRef]
- Akar, B.; Şahin, B. Diversity and ecology of benthic diatoms in Karagöl Lak e in Karagöl-Sahara National Park (Şavşat, Artvin, Turkey). Turk. J. Fish. Aquat. Sci. 2017, 17, 15–24. [Google Scholar] [CrossRef]
- Taş, B.; Hamzaçebi, E.Ş. Assessment of algal diversity and hydrobiological preliminary results in a high-mountain lake (Karagöl Lake, Giresun Mountains, Turkey). Rev. Hydrobiol. 2020, 13, 11–38. (In Turkish) [Google Scholar]
- Wehr, J.D.; Sheath, R.G. Freshwater Algae of North America (Ecology and Classification), 1st ed.; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süβwasser-Benthos von Mitteleuropa, 1st ed.; Koeltz Scientific Books: Königstein, Germany, 2013. [Google Scholar]
- Alles, E.; Nörpel-Schemp, M.; Lange-Bertalot, H. Zur systematik und ökologie charakteristischer Eunotia-Arten (Bacillariophyceae) in elektrolytarmen Bachoberlaufen. Nova Hedwig. 1991, 53, 171–213. [Google Scholar]
- Ortiz-Lerin, R.; Cambra, J. Distribution and taxonomic notes of Eunotia Ehrenberg 1837 (Bacillariophyceae) in rivers and streams of the Northern Spain. Limnetica 2007, 26, 415–434. [Google Scholar] [CrossRef]
- Şahin, B. A new record for the freshwater algal flora of Turkey. Bağbahçe Bilim Derg. 2021, 8, 27–31. [Google Scholar]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherland J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Bjork, S. Redevelopment of lake system—A case study approach. Ambio 1988, 17, 90–98. [Google Scholar]
- Lotter, A.F.; Pienitz, R.; Schmidt, R. Diatoms as indicators of environmental change near arctic and alpine treeline. In The Diatoms: Application for the Environmental and Earth Sciences, 1st ed.; Stoermer, E.F., Smol, J.P., Eds.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Protasov, A.; Barinova, S.; Novoselova, T.; Sylaieva, A. The Aquatic Organisms Diversity, Community Structure, and Environmental Conditions. Diversity 2019, 11, 190. [Google Scholar] [CrossRef] [Green Version]
- Niyatbekov, T.; Barinova, S. Bioindication of aquatic habitats with diatom algae in the Pamir Mountains, Tajikistan. MOJ Ecol. Environ. Sci. 2018, 3, 117–120. [Google Scholar]
- Barinova, S.; Naiz, A.; Barkatullah, S.F. Ecological adaptation to altitude of algal communities in the Swat Valley (Hindu Cush Mountains, Pakistan). Expert Opin. Environ. Biol. 2013, 2, 2. [Google Scholar] [CrossRef]
- Șahin, B.; Akar, B.; Barinova, S. Bioindication of water quality by diatom algae in high mountain lakes of the Natural Park of Artabel Lakes (Gümüşhane, Turkey). Transylv. Rev. Syst. Ecol. Res. Wetl. Divers. 2020, 22, 1–28. [Google Scholar] [CrossRef]











| Variable | Avusor Great Lake | Koçdüzü Great Lake |
|---|---|---|
| North | 40°56′11″ | 41°00′15″ |
| East | 41°12′01″ | 41°11′53″ |
| Altitude | 2678 | 2382 |
| Temperature (°C) | 15.9 | 21.0 |
| Dissolved oxygen (mg L−1) | 10.2 | 9.2 |
| pH | 7.58 | 8.45 |
| Conductivity (μS cm−1) | 45.3 | 104.7 |
| Total Dissolved Matter (mg L−1) | 28.01 | 6.88 |
| Potassium (mg/L) | 0.18 | 0.23 |
| Total Hardness CaCO3 (mg L−1) | 18.96 | 12.94 |
| Calcium (mg L−1) | 5.53 | 3.12 |
| Magnesium (mg L−1) | - | - |
| Ammonium (mg L−1) | 0.14 | 0.16 |
| Chloride (mg L−1) | 6.31 | 10.78 |
| Nitrate (mg L−1) | 1.092 | - |
| Nitrite (mg L−1) | 0.083 | 0.081 |
| Ammonium nitrogen (mg L−1) | 0.11 | 0.12 |
| Nitrate nitrogen (mg L−1) | 0.247 | - |
| Nitrite nitrogen (mg L−1) | 0.025 | 0.024 |
| Medium phosphate (mg L−1) | 0.07 | 0.072 |
| Phosphate (P2O5) (mg L−1) | 0.053 | 0.054 |
| Taxa | Epipelic | Epilithic | Epiphytic | AGL | KGL | Hab | Oxy | pH | pH Range | Sal | T | Tro | Aut-Het | D | Sap | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achnanthidium minutissimum (Kützing) Czarnecki | 1 | 2 | 0 | P-B | st-str | ind | 4.3–9.2 | i | eterm | e | ate | es | b | 0.95 | ||
| Amphora ovalis (Kützing) Kützing | 1 | 2 | 2 | B | st-str | alf | 6.2–9.0 | i | temp | e | ate | sx | b | 1.50 | ||
| Aulacoseira italica (Ehrenberg) Simonsen | 1 | 2 | 0 | P-B | st-str | ind | 5.8–8.4 | i | cool | me | ate | es | b | 1.45 | ||
| Aulacoseira lacustris (Grunow) Krammer | 1 | 0 | 2 | P | - | acf | - | hb | cool | ot | - | - | x-o | 0.50 | ||
| Aulacoseira valida (Grunow) Krammer | 1 | 0 | 2 | P-B | - | alf | - | i | - | om | ate | es | o | 1.30 | ||
| Aulacoseira sp. | 1 | 0 | 2 | - | - | - | - | - | - | - | - | - | - | - | ||
| Brachysira brebissonii R.Ross | 1 | 0 | 2 | P-B | st-str | acf | 4.6–7.8 | hb | temp | ot | ats | sx | o | 0.40 | ||
| Caloneis alpestris (Grunow) Cleve | 1 | 2 | 0 | B | str | alf | - | i | - | m | ats | - | o | 0.10 | ||
| Caloneis silicula (Ehrenberg) Cleve | 1 | 2 | 0 | B | st | ind | 6.3–9.0 | i | warm | om | ats | sp | o | 1.30 | ||
| Cocconeis placentula var. euglypta (Ehrenberg) Grunow | 1 | 2 | 0 | P-B | st-str | alf | 5.5–9.0 | i | temp | om | ate | sx | b | 1.30 | ||
| Cymbella affinis Kützing | 1 | 2 | 0 | B | st-str | alf | 6.3–9.5 | i | temp | e | ats | sx | b | 1.10 | ||
| Cymbella cistula (Ehrenberg) O.Kirchner | 1 | 1 | 2 | 0 | B | st-str | alf | 8.0 | i | - | e | ats | sx | b | 1.20 | |
| Cymbella sp. | 1 | 2 | 0 | - | - | - | - | - | - | - | - | - | - | - | ||
| Cymbopleura amphicephala (Nägeli ex Kützing) Krammer | 1 | 1 | 0 | 2 | B | st-str | ind | 4.6–8.10 | i | - | om | ats | sx | o | 1.20 | |
| Diatoma vulgaris Bory | 1 | 2 | 2 | P-B | st-str | alf | 6.2–8.9 | i | temp | me | ate | sx | b | 2.20 | ||
| Diatoma sp. | 1 | 2 | 0 | - | - | - | - | - | - | - | - | - | - | - | ||
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | 1 | 1 | 2 | 0 | B | st-str | ind | - | i | - | ot | ate | sx | o-x | 0.70 | |
| Diploneis elliptica (Kützing) Cleve | 1 | 2 | 0 | B | str | alf | 8.2 | i | temp | m | ats | sx | o-x | 0.60 | ||
| Encyonema minutum (Hilse) D.G.Mann | 1 | 1 | 1 | 0 | B | st-str | ind | 4.9–8.9 | i | temp | m | ate | sx | o | 1.20 | |
| Encyonema silesiacum (Bleisch) D.G.Mann | 1 | 2 | 0 | B | st-str | ind | 6.2–8.6 | i | temp | o-e | ate | sx | o | 1.20 | ||
| Epithemia gibba (Ehrenberg) Kützing | 1 | 0 | 2 | P-B | st-str | alf | 6.2–9.0 | i | temp | om | ate | es | x-o | 1.40 | ||
| Eunotia ambivalens Lange-Bertalot & Tagliaventi | 1 | 0 | 2 | B | - | acf | - | i | - | ot | - | - | o | 1.00 | ||
| Eunotia arcus Ehrenberg | 1 | 0 | 2 | B | st-str | acf | 5.8–6.95 | i | temp | ot | ats | - | x-o | 0.50 | ||
| Eunotia bilunaris (Ehrenberg) Schaarschmidt | 1 | 0 | 2 | B | st-str | acf | 5.0–7.8 | i | temp | o-e | ate | es | o | 1.00 | ||
| Eunotia diodon Ehrenberg | 1 | 0 | 2 | B | st-str | acf | 6.75 | i | cool | ot | ats | - | x | 0.20 | ||
| Eunotia exigua (Brébisson ex Kützing) Rabenhorst | 1 | 0 | 2 | P-B, aer | st-str | acb | 3.4–8.0 | hb | temp | o-e | ate | es | x-o | 0.45 | ||
| *Eunotia hexaglyphis Ehrenberg | 1 | 0 | 2 | B | - | acf | 5.79–6.66 | hb | temp | - | - | - | o-x | 0.70 | ||
| Eunotia mucophila (Lange-Bertalot, Nörpel-Schempp & Alles) Lange-Bertalot | 1 | 2 | 2 | P-B | st-str | acf | 5.25–6.4 | hb | temp | om | ate | - | o | 1.00 | ||
| Eunotia praerupta Ehrenberg | 1 | 2 | 0 | P-B | st-str | acf | 6.68–8.0 | hb | cool | om | ats | sx | x-o | 0.40 | ||
| Fragilaria capucina Desmazières | 1 | 2 | 2 | P-B | st-str | ind | 6.4–8.9 | i | temp | m | ats | es | b-o | 1.60 | ||
| Frustulia crassinervia (Brébisson ex W.Smith) Lange-Bertalot & Krammer | 1 | 0 | 2 | B | str | acf | 4.7–7.2 | hb | - | ot | ats | es | x-o | 0.50 | ||
| Gomphonella calcarea (Cleve) R.Jahn & N.Abarca | 1 | 2 | 0 | B | st-str | alf | - | i | - | om | ate | b | 2.30 | |||
| Gomphonella olivacea (Hornemann) Rabenhorst | 1 | 2 | 0 | B | st-str | alf | 6.5–8.8 | i | temp | e | ate | es | o-b | 1.45 | ||
| Gomphonema acuminatum Ehrenberg | 1 | 0 | 2 | B | st-str | ind | 6.3–9.5 | i | temp | om | ats | es | o-b | 1.40 | ||
| Gomphonema hebridense W.Gregory | 1 | 0 | 2 | B | - | acf | 6.1 | - | - | - | - | - | - | - | ||
| Gomphonema montanum (Schumann) Grunow | 1 | 2 | 0 | B | str | ind | - | i | - | m | ats | es | x-b | 0.85 | ||
| Gomphonema pala E.Reichardt | 1 | 2 | 0 | B | - | - | - | - | - | - | - | - | o-b | 1.40 | ||
| Gomphonema subclavatum (Grunow) Grunow | 1 | 2 | 0 | B | str | ind | - | i | - | om | ats | es | o-b | 1.40 | ||
| Gomphonema truncatum Ehrenberg | 1 | 3 | 0 | 2 | B | st-str | ind | 7.19 | i | temp | me | ats | es | o-b | 1.40 | |
| Gomphonema sp. | 1 | 2 | 0 | - | - | - | - | - | - | - | - | - | - | - | ||
| Gyrosigma attenuatum (Kützing) Rabenhorst | 1 | 2 | 0 | P-B | st-str | alf | 6.9–8.5 | i | temp | om | ate | - | o-a | 1.80 | ||
| Hannaea arcus (Ehrenberg) R.M.Patrick | 1 | 2 | 3 | 0 | B | str | alf | 5.7–7.5 | i | temp | om | ats | es | x | 0.30 | |
| Iconella linearis (W.Smith) Ruck & Nakov | 1 | 2 | 2 | P-B | st-str | ind | 4.6–9.0 | i | - | om | ats | es | x-o | 0.50 | ||
| Melosira undulata (Ehrenberg) Kützing | 1 | 0 | 2 | P-B | - | ind | - | i | - | me | - | es | b | 2.00 | ||
| Meridion circulare (Greville) C.Agardh | 1 | 2 | 0 | P-B | st-str | ind | 6.6–8.3. | i | temp | om | ate | es | o | 1.10 | ||
| Navicula cryptocephala Kützing | 1 | 2 | 2 | P-B | st-str | ind | 6.5–8.4 | i | temp | o-e | ate | es | b | 2.10 | ||
| Navicula cryptotenella Lange-Bertalot | 1 | 2 | 0 | P-B | st-str | ind | 6.5–8.7 | i | temp | m | ats | es | o | 1.30 | ||
| Navicula minima Grunow | 1 | 2 | 2 | P-B | st-str | alf | 6.7–7.8 | hl | temp | e | hne | es | a-o | 2.60 | ||
| Navicula pseudosilicula Hustedt | 1 | 2 | 0 | P-B | - | ind | - | i | - | ot | - | - | o | 1.00 | ||
| Navicula radiosa Kützing | 1 | 2 | 2 | B | st-str | ind | 5–9 | i | temp | me | ate | es | b | 1.30 | ||
| Neidium ampliatum (Ehrenberg) Krammer | 1 | 0 | 2 | B | - | acf | 8.5–10.5 | hb | temp | ot | - | - | o | - | ||
| Neidium bisulcatum var. subampliatum Krammer | 1 | 2 | 0 | B | - | acf | – | hb | - | - | - | - | - | - | ||
| Neidium dubium (Ehrenberg) Cleve | 1 | 2 | 0 | B | str | alf | – | i | - | me | ats | - | b-o | 1.70 | ||
| Nitzschia fonticola (Grunow) Grunow | 1 | 2 | 0 | P-B | st-str | alf | 6.0–8.9 | i | temp | me | ate | - | o-b | 1.50 | ||
| Nitzschia sublinearis Hustedt | 1 | 2 | 0 | P-B | - | alf | – | i | - | me | hne | es | a | 3.00 | ||
| Odontidium hyemale (Roth) Kützing | 1 | 0 | 2 | P-B | st-str | ind | 6.5–7.5 | hb | cool | ot | ats | sx | x | 0.30 | ||
| Odontidium mesodon (Kützing) Kützing | 1 | 2 | 0 | B | st-str | ind | 6.6–8.3 | hb | cool | ot | ats | sx | x-o | 0.40 | ||
| Orthoseira dendroteres (Ehrenberg) Genkal & Kulikovskiy | 1 | 2 | 0 | B, aer | - | - | – | i | - | - | - | - | x-o | 0.50 | ||
| Pinnularia borealis Ehrenberg | 1 | 2 | 2 | B, aer | st-str, aer | ind | 7.8 | i | om | ate | es | x-o | 0.40 | |||
| Pinnularia brebissonii (Kützing) Rabenhorst | 1 | 0 | 2 | B | st-str | ind | - | i | temp | - | ats | - | p-a | - | ||
| Pinnularia interrupta W.Smith | 1 | 2 | 2 | B | st-str | ind | 6.0–8.0 | i | - | om | ats | - | o | - | ||
| Pinnularia major (Kützing) Rabenhorst | 1 | 1 | 1 | 2 | 2 | B | st-str | ind | 6.38–7.1 | i | temp | me | ate | o-x | 0.60 | |
| Pinnularia microstauron var. nonfasciata Krammer | 1 | 0 | 2 | B | - | - | - | - | - | - | - | - | - | - | ||
| Pinnularia viridis (Nitzsch) Ehrenberg | 1 | 2 | 0 | P-B | st-str | ind | 5.24–7.1 | i | temp | o-e | ate | es | x | 0.30 | ||
| Stauroneis anceps Ehrenberg | 1 | 3 | 2 | 2 | P-B | st-str | ind | 4.8–8.0 | i | - | om | ate | sx | o | 1.30 | |
| Staurosira construens Ehrenberg | 1 | 2 | 0 | P-B | st-str | alf | 5.5–9.0 | i | temp | me | ats | sx | o | 1.30 | ||
| Staurosirella pinnata (Ehrenberg) D.M.Williams & Round | 1 | 2 | 0 | P-B | st-str | alf | 6.2–9.3 | hl | temp | o-e | ate | es | o | 1.20 | ||
| Surirella angusta Kützing | 1 | 2 | 0 | P-B | st-str | alf | 6.9–8.9 | i | temp | e | ate | es | b-o | 1.70 | ||
| Surirella robusta Ehrenberg | 1 | 2 | 0 | P-B | st-str | ind | 7.6–9.5 | i | temp | ot | ats | es | o | 1.20 | ||
| Tabellaria fenestrata (Lyngbye) Kützing | 1 | 3 | 0 | 2 | P-B | st-str | ind | 6.2 | i | - | om | ats | es | x | 0.30 | |
| Tabellaria flocculosa (Roth) Kützing | 1 | 3 | 1 | 1 | P-B | st-str | acf | 4.5–8.0 | i | eterm | ot | ats | es | o-x | 0.60 |
| Lake | Altitude, m a.s.l. | Temperature, °C | DO, mg L−1 | pH | Conductivity, µSm cm−1 | No Species |
|---|---|---|---|---|---|---|
| Poyrazlar | 30 | 14.6 | 6.98 | 7.90 | 425 | 44 |
| Mogan | 972 | 14.3 | 11.50 | 8.80 | 258 | 41 |
| Abant | 1350 | 16.5 | 8.80 | 8.25 | 264 | 41 |
| Karagol | 1512 | 18.6 | 6.90 | 8.01 | 138.5 | 76 |
| KGL | 2382 | 21.0 | 9.20 | 8.45 | 104.7 | 34 |
| IL | 2668 | 19.1 | 4.25 | 6.78 | 12.0 | 38 |
| AGL | 2678 | 15.9 | 10.2 | 7.58 | 45.3 | 46 |
| ACL | 2712 | 16.2 | 2.80 | 7.20 | 32.3 | 43 |
| ARL | 2834 | 15.3 | 6.60 | 7.00 | 33.4 | 53 |
| BL | 2883 | 13.0 | 9.00 | 7.00 | 26.0 | 46 |
| YLP | 2980 | 14.5 | 2.34 | 7.20 | 29.2 | 21 |
| YL | 2980 | 13.4 | 2.80 | 6.90 | 26.6 | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, B.; Barınova, S. Role of Altitude in Formation of Diatom Diversity of High Mountain Protected Glacier Lakes in the Kaçkar Mountains National Park, Rize, Turkey. Environments 2022, 9, 127. https://doi.org/10.3390/environments9100127
Şahin B, Barınova S. Role of Altitude in Formation of Diatom Diversity of High Mountain Protected Glacier Lakes in the Kaçkar Mountains National Park, Rize, Turkey. Environments. 2022; 9(10):127. https://doi.org/10.3390/environments9100127
Chicago/Turabian StyleŞahin, Bülent, and Sophia Barınova. 2022. "Role of Altitude in Formation of Diatom Diversity of High Mountain Protected Glacier Lakes in the Kaçkar Mountains National Park, Rize, Turkey" Environments 9, no. 10: 127. https://doi.org/10.3390/environments9100127
APA StyleŞahin, B., & Barınova, S. (2022). Role of Altitude in Formation of Diatom Diversity of High Mountain Protected Glacier Lakes in the Kaçkar Mountains National Park, Rize, Turkey. Environments, 9(10), 127. https://doi.org/10.3390/environments9100127